Investigations of the secondary metabolites of microorganisms isolated from unusual or specialized ecological niches may increase the chances of discovering bioactive or potential lead compounds. Endophytes are microorganisms (including fungi, bacteria and actinomycete) that spend the whole or part of their life cycle colonizing inter- and/or intra-cellularly inside the healthy tissues of the host plant,but generally are nonpathogenic in nature [1, 2].
From the results reported before,it was concluded that endophytes produce a variety of bioactive compounds originally characteristic of the host or some chemically novel compounds generated by unusual biosynthetic routes [3, 4, 5, 6]. Obviously, endophytes are a rich source of bioactive and novel compounds with huge medicinal and agricultural potential. As a result, investigations of secondary metabolites from endophytes have attracted substantial interest of many researchers who aspire to identify lead compounds for new drugs. As part of our ongoing work for searching novel bioactive natural compounds from endophytic fungi of medicinal plants [7, 8, 9, 10],more than 40 fungal strains were isolated from the medicinal plantAnnonam muricatadistributed in Hainan province,China. Bioassay-guided fractionation of the ethyl acetate extract of the fermentation broth from one of the endophytic fungus,Penicilliumsp. F-14,led to the isolation of a new nonadride derivative rubratoxin C (1) together with a known compound rubratoxin B (2). Rubratoxin B is a mycotoxin with potent hepatotoxicity produced by various Penicillium fungi [11, 12]. However,it was reported that rubratoxin B has anti-tumor activity,which can restrain the cell cycle progression of mammary carcinoma cells and possess cytotoxic activity against human fibrosarcoma HT1080 and Yoshida ascites sarcoma cells [13, 14]. In this communication,we describe the isolation,structural elucidation and anti-tumor activity of rubratoxin B and rubratoxin C isolated fromPenicilliumsp. F-14. 2. Experimental
Optical rotations were determined using a PE Model 343 spectrometer. IR spectra were obtained on a Thermo Nicolet 5700 spectrometer.1H NMR and 13C NMR spectra were recorded on a Bruker INOVA-500 spectrometer (500 MHz for 1H NMR and 125 MHz for 13C NMR). HR-ESI-MS were measured on a Q-trap ESI mass spectrometer (Applied Biosystems/MDS Sciex,Carlsbad, CA,USA). Semi-prep. HPLC were performed using Apollo Silica (250 mm × 10.0 mm i.d.,5μm) or Grace Adsorbosphere C18 (250 mm × 10.0 mm i.d.,5μm) columns. Column chromatography was carried out using Sephadex LH-20 (Pharmacia,Sweden) and silica gel (300-400 mesh,Qingdao Haiyang Chemical Co.,Ltd., China). 2.1. Strain isolation and identification
The strain ofPenicilliumsp. F-14 was isolated from the fruit ofA. muricataand identified as a new species ofPenicilliumsp. based on morphology analysis and molecular identification by China Center of Industrial Culture Collection. Phylogenetic analysis of ITS sequences indicated that the strain belongs to a distinct branch and bootstrap support reached up to 99%. Besides,the homology between Penicilliumsp. F-14 and other known stains was less than 96.5%. 2.2. Strain fermentation
Microbial cultures were grown according to the standard twostage fermentation protocol. A slant culture ofPenicilliumsp. F-14 was inoculated into 250 mL flasks with 50 mL of potato dextrose medium shaking at 260 rpm and 288C for 3 days and used as seed cultures. A volume of 5 mL seed cultures were transferred to a 500 mL flask containing 175 mL of medium and cultivated under the same conditions for additional 7 days. 2.3. Extraction and isolation
After 7 days’ culturing,the fermentation broth (80 L) ofPenicillium sp. F-14 was filtered under reduced pressure. The filtrate was extracted with ethyl acetate for 3 times and the ethyl acetate layer was evaporated under vacuum to afford a residue (29.98 g). The dry mycelia were extracted 3 times in an ultrasonic bath by ethyl acetate, and the resulting extract was concentrated under vacuum to yield a residue (13.27 g). Considering that the above two parts of extract showed similar anti-tumor activity and TLC appearance,they were combined and subjected to silica gel column chromatography. The CHCl3-MeOH gradient mixtures (10:1,5:1,1:1 and 0:1) were used as the eluent to afford 4 fractions (A-D) based on the TLC analysis. Fraction B (12.56 g) was further fractionatedviasilica gel column chromatography eluting with petroleum ether-acetone (from 5:1 to 0:1) to produce 4 sub-fractions. Sub-fraction 2 was then subjected to reverse-phase semi-prep. HPLC using acetonitrile-H2O (40:60) as mobile phase to give rubratoxin C (1,6.4 mg) and rubratoxin B (2, 24.9 mg). Moreover,it was notable that further isolation yielded 6.5 g of rubratoxin B from sub-fraction 3. 3. Results and discussion
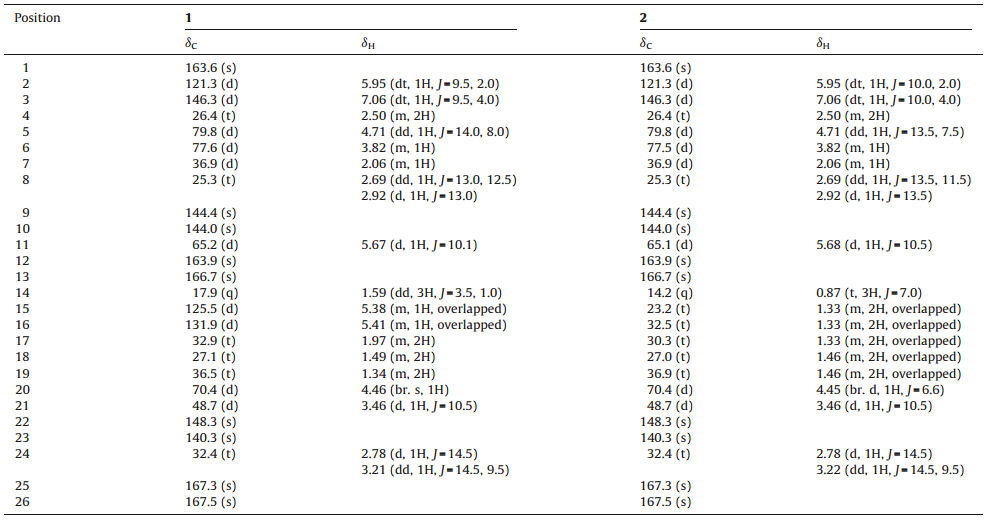
Under the guidance of anti-tumor activity analysis,various chromatographic techniques wereemployed to isolate and purify the ethyl acetate extract of the Penicilliumsp. F-14 fermentation. Consequently,one new compound rubratoxin C (1)togetherwitha knownanti-tumor compoundrubratoxin B(2) were obtained,andtheir structures were determined by detailed spectroscopic analysis (Fig. 1).
|
Download:
|
| Fig. 1.The structures of 1 and 2. | |
Compound 1 was obtained as white amorphous powder;[α]D20+45.8 (c 0.12,MeOH). Its molecular formula was assigned as C26H28O11 with 13 degrees of unsaturation by HR-ESI-MS (m/z 517.1731,[M+H]+ ,calcd.for C26H29O11 517.1710),as well as 1H NMR, 13C NMR and DEPT spectroscopic data analyses (Fig. S1-S3 in Supporting information). The 13C NMR data revealed the presence of 26 carbon signals including 9 quaternary carbons,10 methines,6 methylenes,and 1 methyl. Further analysis of the infrared (IR) absorption at 1847 cm -1 (w),1828 cm -1 (w),1766 cm -1 (vs), 1708 cm -1 (vs) and 1260 cm -1 (s) suggested the presence of two dialkylmaleic anhydride functional groups in the molecule (Fig. S4 in Supporting information). The presence of an unsaturated δ-lactone moiety was confirmed by the NMR data [Table 1,δH 7.06 (dt, 1H,J=9.5,4.0 Hz,H-3)/δC 146.3;δH 5.95 (dt,1H,J=9.5,2.0Hz,H-2)/ δC 121.3;δH 4.71 (dd,1H,J= 14.0,8.0 Hz,H-5)/δC 79.8; a multiplet methylene at δH 2.50 (m,2H,H-4)/δC 26.4,and one quaternary carbons at δC163.6]. The NMR signals at δH5.38 (m,1H,overlapped)/ δC 125.5 and δH5.41 (m,1H,overlapped)/δC 131.9 indicated the presence of one alkyl double bond. These accounted for 12 of 13 degrees of unsaturation required by the molecular formula,which suggested there would be another ring in 1.The 1 H NMR and 13 C NMR spectroscopic data of compound 1 are shown in Table 1.All these evidence indicated that 1 may be a nonadride derivative, which has a unique nine-membered carbocyclic ring with an affixed dialkylmaleic anhydride unit. Through extensive analysis,it was found that the NMR spectrum of 1 was similar to that of 2,which was a nonadride derivative isolated together with 1 and identified as rubratoxin B by comparing its NMR data with those in the literature [15]. The 1 H NMR spectrum of 1 was very similar to that of 2,except additional methine signals at δH 5.38 (H-15) and δH 5.41 (H-16) in 1, while the methylene proton signals at δH 1.33 (m,6H,H-15,H-16,H-17) was not observed,suggesting the presence of an alkyl double bond in 1. This was further supported by the observation that the signals of C-15 and C-16 in 1 shifted downfield to δC 125.5 (d) and δC 131.9 (d) from δC 23.2 (t) and δC 32.5 (t) in 2,respectively.
| Table 1 1H NMR and 13C NMR data of 1 and 2(acetone-d6,δ in ppm,J in Hz) |
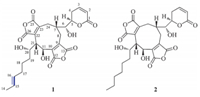
All those discussed above confirmed the structure of 1,which was characterized as a new nonadride derivative named rubratoxin C. The structure of 1 was further confirmed by HMQC and HMBC spectra (Fig. 2,Figs. S5 and S6 in Supporting information). The stereochemistry of nonadrides was studied extensively in the past few decades and the absolute configurations of rubratoxin B was unambiguously defined [16, 17, 18]. The stereochemistry of double bond [C-15(16)] in 1 was assigned asEconfiguration by analysis of 1D NOE difference spectra (Fig. S7 in Supporting information). When irradiating H-14 ( δH 1.59),the enhancement of H-16 ( δH 5.41) was observed. Besides,H-15 ( δH 5.38) was enhanced when H-17 ( δH 1.97) was irradiated,while the enhancement of H-14 ( δH 1.59) was not observed.
|
Download:
|
| Fig. 2.The key HMBC correlations of 1. | |
The compounds 1 and 2 were tested for the cytotoxicity using the MTT method. Interestingly,primary bioassays showed that 2 had moderate cytotoxic effect against HCT-8,BEL-7402,Ketr3, A2780,MCF-7 and BGC-823 human cancer cells,with IC50 values of 27.31,26.75,33.08,26.42,29.39 and 33.81 μmol/L,respectively. However,compound 1 exhibited weak activity,and the IC50 values were all greater than 100 μmol/L. 4. Conclusion
Endophytes can provide a rich source of bioactive and chemically novel compounds with huge medicinal and agricultural potential. In this article,based on the IR,MS,1D NMR and 2D NMR spectroscopic data and comparison with those reported in the literature,a new nonadride derivative rubratoxin C (1) together with a known compound rubratoxin B (2) were isolated and identified from the endophytic fungus Penicilliumsp. F-14. The results suggested that structurally diverse nonadrides existed in Penicilliumsp. F-14. Considering the cytotoxicity against tumor cells and the high yield of rubratoxin B as a potent mycotoxin, further structural optimization would be of great significance for searching potential anti-tumor leads.
AcknowledgmentsThis work was financially supported by the Science & Technology Project of Guangdong Province (No. 2011A080403020),the Fundamental Research Funds for the Central Universities (No. 2012N06), and the National Science & Technology Major Project ‘Key New Drug reation and Manufacturing’,China (No. 2012X09301002-001-005).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.03.040
| [1] | H.W. Zhang, Y.C. Song, R.X. Tan, Biology and chemistry of endophytes, Nat. Prod. Rep. 23 (2006) 753-771. |
| [2] | R.X. Tan, W.X. Zou, Endophytes: a rich source of functional metabolites, Nat. Prod. Rep. 18 (2001) 448-459. |
| [3] | S. Kaul, S. Gupta, M. Ahmed, M.K. Dhar, Endophytic fungi from medicinal plants: a treasure hunt for bioactive metabolites, Phytochem. Rev. 11 (2012) 487-505. |
| [4] | Wuringege, Z.K. Guo, W. Wei, et al., Polyketides from the plant endophytic fungus Cladosporium sp. IFB3lp-2, J. Asian Nat. Prod. Res. 15 (2013) 928-933. |
| [5] | Z.F. Fang, S.S. Yu, W.Q. Zhou, et al., A new isocoumarin from metabolites of the endophytic fungus Alternaria tenuissima (Nees & T. Nees: Fr.) Wiltshire, Chin. Chem. Lett. 23 (2012) 317-320. |
| [6] | A. Stierle, G. Strobel, D. Stierle, Taxol and taxane production by Taxomyces andreanae, an endophytic fungus of Pacific yew, Science 260 (1993) 214-216. |
| [7] | J.M. Wang, G.Z. Ding, L. Fang, et al., Thiodiketopiperazines produced by the endophytic fungus Epicoccum nigrum, J. Nat. Prod. 73 (2010) 1240-1249. |
| [8] | J.M. Wang, N. Jiang, J. Ma, et al., Study on absolute configurations of a/a0 chiral carbons of thiodiketopiperazines by experimental and calculated circular dichroism spectra, Tetrahedron 69 (2013) 1195-1201. |
| [9] | H.L. Ge, D.W. Zhang, L. Li, et al., Two new terpenoids from endophytic fungus Periconia sp. F-31, Chem. Pharm. Bull. 59 (2011) 1541-1544. |
| [10] | D.W. Zhang, H.L. Ge, D. Xie, et al., Periconiasins A-C, new cytotoxic cytochalasans with an unprecedented 9/6/5 tricyclic ring system from endophytic fungus Periconia sp., Org Lett. 15 (2013) 1674-1677. |
| [11] | S. Natori, S. Sakaki, H. Kurata, et al., Production of rubratoxin B by Penicillium purpurogenum Stoll, Appl. Microbiol. 19 (1970) 613-617. |
| [12] | H. Nagashima, K. Nakamura, T. Goto, Stress-activated MAP kinases regulate rubratoxin B-caused cytotoxicity and cytokine secretion in hepatocyte-derived HepG2 cells, Toxicol. Lett. 155 (2005) 259-267. |
| [13] | T. Wang, Y. Zhang, Y. Wang, Y.H. Pei, Anti-tumor effects of rubratoxin B on cell toxicity, inhibition of cell proliferation, cytotoxic activity and matrix metalloproteinase-2,9, Toxicol. in Vitro 21 (2007) 646-650. |
| [14] | V. Fimiani, A. Richetti, Antitumor effect of a mycotoxin: rubratoxin B, Chemotherapy 39 (1993) 59-62. |
| [15] | S. Nieminen, C. Tamm, 1H-und 13C-NMR spektroskopie der nonadride, Helv. Chim. Acta 64 (1981) 2791-2801. |
| [16] | G. Buechi, K.M. Snader, J.D. White, J. Zanos Gougoutas, S. Singh, Structures of rubratoxins A and B, J. Am. Chem. Soc. 92 (1970) 6638-6641. |
| [17] | J.D. White, J. Kim, N.E. Drapela, Enantiospecific synthesis of (+)-byssochlamic acid, a nonadride from the ascomycete Byssochlamys fulva, J. Am. Chem. Soc. 122 (2000) 8665-8671. |
| [18] | Y.B. Liu, G.Z. Ding, Y. Li, et al., Structures and absolute configurations of penicillactones A-C from an endophytic microorganism, Penicillium dangeardii Pitt, Org. Lett. 15 (2013) 5206-5209. |