Nickel-catalyzed cross-coupling reactions represent a versatile tool for the C-X (X = C,N,O,etc.) bond formation with aryl halides and analogs [1, 2, 3, 4, 5, 6]. The palladium mediated Sonogashira coupling reaction [7] is one of the most widely used reactions in organic synthesis [8, 9, 10, 11, 12] for the preparation of potential bioactive compounds [13, 14],new materials [15, 16, 17],and natural products [18, 19, 20],but the same transformation catalyzed by nickel has not been explored much. In the past decade,researchers have directed their efforts toward the development of more efficient or single metal catalyst systems with milder reaction conditions and other desirable attributes [19, 20, 21, 22, 23, 24, 25]. While these efforts have provided alternative methods for the synthesis of alkynes,the development of rapid and more efficient procedures for the preparation of alkynes by Sonogashira coupling still remains a challenge. Aryl halides,especially aryl iodides and bromides,and alkynes are the preferred coupling partners in these reactions. Particularly, (bromoethynyl)benzene (1a),which is easily synthesized from 1-ethynylbenzene,has been studied in cross-coupling reactions [26, 27, 28, 29].
Grignard reagents are reactive nucleophilic reagents and can easily be prepared from the corresponding halides [30, 31, 32]. Although the synthesis of 1,2-disubstituted alkynes by coppercatalyzed cross-coupling of Grignard reagents with alkynyl bromide has been described [33, 34],the synthesis based on direct coupling of alkynyl halide with Grignard reagents using nickel catalysts has been rarely studied. Herein,we report a new method for the synthesis of 1,2-disubstituted alkynesviaNickel-catalyzed cross-couplings between (bromoethynyl)benzene (1a) and Grignard reagents in the presence of NiCl2 (4 mol%) and (p-CH3Ph)3P (8 mol%) at room temperature. 2. Experimental
1H NMR and 13C NMR spectra were recorded on a Varian 400 MHz spectrometer. The chemical shifts are reported relative to Me4Si. Analytical thin-layer chromatography (TLC) was performed on silica gel 60 F254 plates. Flash column chromatography was carried out on silica gel (200-400 mesh). All reactions were carried out under a nitrogen atmosphere. The starting material (bromoethynyl)benzene was prepared according to literature procedures [35]. Chemical reagents and solvents were purchased from Aldrich or Alfa Aesar,and were used without further purification with the exception of the following reagents: THF,Et2O,hexane and toluene were distilled from sodium under nitrogen,and dichloromethane was distilled from CaH2. Mass spectra were carried out on a Finnigan MAT-4510 spectrometer.
General procedure for cross-coupling of (bromoethynyl)benzene with Grignard reagent: Under an atmosphere of nitrogen, NiCl2 (6.6 mg,0.04 mmol),(p-CH3Ph)3P (0.08 mmol),and THF (2 mL) were mixed in a Schlenk flask. Shortly afterwards,the reaction mixture was cooled to 0 ℃ and a solution of ArylMgBr or AlkylMgBr (1.3 mmol) was added with a syringe pump,and subsequently the (bromoethynyl) benzene was added. After the addition,the reaction mixture was allowed to stir for an additional 3-4 h at room temperature. After the completion of the reaction, the reaction mixture was diluted with 1 mol/L aqueous HCl solution (10 mL) and extracted with Et2O (15 mL × 3). The combined organic layers were dried over anhydrous Na2SO4, filtered and evaporatedin vacuo. The residue was subjected to flash column chromatography on silica gel (hexane gradient) to afford the corresponding products.
Diphenylacetylene (3aa) [36]. White solid,mp 60-61 ℃ (lit. [35] 59-60 ℃). 1H NMR (400 MHz,CDCl3): δ 7.56-7.52 (m,4H), 7.36-7.33 (m,6H); 13C NMR (100 MHz,CDCl3): δ 131.2,128.3, 128.2,123.4,89.3; EI-MS (m/z): 178 (M+).
1-Phenyl-2-(o-tolyl)acetylene (3ab) [36]. Colorless oil. 1H NMR (400 MHz,CDCl3):δ 7.56-7.48 (m,3H),7.38-7.31 (m,3H),7.24- 7.14 (m,3H),2.52 (s,3H); 13C NMR (100 MHz,CDCl3): δ 140.2, 131.8,131.5,129.4,128.3,128.1,125.6,123.5,123.0,93.3,88.3, 20.7; EI-MS (m/z): 192 (M+).
1-Phenyl-2-(m-tolyl)acetylene (3ac) [23]. Colorless oil. 1H NMR (400 MHz,CDCl3):δ 7.60-7.48 (m,2H),7.45-7.30 (m,5H),7.24(t,1H,J=7.6Hz),7.06(d,1H,J= 7.6 Hz),2.35 (s, 3H). 13C NMR (100 MHz,CDCl3):δ 138.1,132.3,131.7,129.3, 128.8,128.4,128.3,128.3,121.7,121.5,89.7,89.1,21.4; EI-MS (m/z): 192 (M+ ).
1-Phenyl-2-(p-tolyl)acetylene (3ad) [23]. White solid,mp 70- 71 ℃ (lit. [23] 71-72 ℃). 1H NMR (400 MHz,CDCl3):δ 7.54-7.49 (m,2H),7.43 (d,2H,J= 8.1 Hz),7.37-7.30 (m,3H),7.14 (d,2H, J= 8.1 Hz),2.36 (s,3H); 13C NMR (100 MHz,CDCl3):δ 138.4,131.5, 131.4,129.1,128.3,128.0,123.4,120.1,89.5,88.7,21.5; EI-MS (m/z): 192 (M+ ).
1-Phenyl-2-(m-methoxyphenyl)acetylene (3ae) [37]. 1H NMR (400 MHz,CDCl3):δ 7.54-7.57 (m,2H),7.35-7.37 (m,1H),7.25- 7.29 (m,3H),7.15 (d,1H,J= 8.0 Hz),7.08 (s,1H),6.90-6.92 (m,1H), 3.84 (s,1H); 13C NMR (100 MHz,CDCl3): δ 159.3,131.6,129.4, 128.33,128.28,124.24,124.16,123.2,116.3,114.9,89.3,89.2, 55.3; EI-MS (m/z): 208 (M+ ).
1-Phenyl-2-(p-methoxyphenyl)acetylene (3af) [36]. White solid,mp 55-56 ℃ (lit. [36] 55 ℃). 1H NMR (400 MHz,CDCl3): δ 7.53 -7.45 (m,4H),7.37-7.26 (m,3H),6.88 (d,2H,J=9.0Hz), 3.82 (s,3H); 13C NMR (100 MHz,CDCl3): δ 159.6,133.0,131.4, 128.3,127.9,123.6,115.4,114.0,89.3,88.0,55.3; EI-MS (m/z): 208 (M+ ).
1-(3,5-Dimethylphenyl)-2-phenylacetylene(3ag)[36]. White solid,mp 44-45 ℃ (lit. [36] 45 ℃). 1H NMR (400 MHz,CDCl3):δ 7.55-7.43 (m,2H),7.36-7.27 (m,3H),7.30 (s,2H),7.15 (s,1H), 2.28 (s,6H). 13C NMR (100 MHz,CDCl3): δ 138.1,132.3,131.7, 129.3,128.8,128.4,128.3,128.3,89.7,89.1,21.4; EI-MS (m/z): 206 (M+ ).
1-Phenyl-2-(p-chloro)acetylene (3ah) [36]. White solid,mp 82- 83 ℃ (lit. [36] 84 ℃). 1H NMR (400 MHz,CDCl3):δ 7.54-7.51 (m, 2H),7.47-7.45 (d 2H,J= 8.4 Hz,),7.37-7.31 (m,5H); 13C NMR (100 MHz,CDCl3):δ 134.2,132.8,131.6,128.6,128.4,128.0,122.9, 121.7,90.3,88.2; EI-MS (m/z): 212 (M+ ).
1-Phenyl-2-(p-floro)acetylene (3ai)[36]. Pale yellow solid,mp 109-110 ℃ (lit. [36] 110 ℃). 1H NMR (400 MHz,CDCl3):δ 7.60- 7.45 (m,4H),7.40-7.32 (m,3H),7.08-7.00 (m,2H); 13C NMR (100 MHz,CDCl3): δ 164.3,161.0,133.6 (d,J= 8.60 Hz),131.7, 128.5 (d,J= 2.87 Hz),123.2,119.5,115.7 (d,J= 21.5 Hz),89.1,88.4; EI-MS (m/z): 196 (M+ ).
1-Phenyl-2-(p-trifloromethyl)acetylene (3aj) [38]. White solid,mp 125-126 ℃. 1H NMR (400 MHz,CDCl3): δ 7.61-7.64 (m, 4H),7.55-7.59 (m,2H),7.36-7.39 (m,3H); 13C NMR (100 MHz, CDCl3): δ 131.9,131.8,129.9 (J= 32.4 Hz),128.9,128.5,127.1 (J= 1.4 Hz),125.2 (J= 3.9 Hz),123.9 (J= 270.1 Hz),122.6,91.8, 87.9; EI-MS (m/z): 246 (M+ ).
1-Phenyl-2-(p-trimethylsilane)acetylene (3ak) [24]. White solid,mp 119-121 ℃. 1H NMR (400 MHz,CDCl3): δ 7.57-7.53 (m,6H),7.38-7.35 (m,3H),7.14 (d,2H,J= 8.1 Hz),2.36 (s,3H); 13C NMR (100 MHz,CDCl3): δ 141.0,133.2,131.6,130.6,129.2, 128.4,128.3,123.5,89.5,88.7,2.5; EI-MS (m/z): 250 (M+ ).
1-(1-Naphthyl)-2-phenylacetylene (3al) [24]. Colorless oil. 1H NMR (400 MHz,CDCl3): δ 8.45 (d,1H,J= 7.5 Hz),7.85 (t,2H, J= 8.1 Hz),7.77 (dd,1H,J= 7.2 Hz,1.2 Hz),7.67-7.63 (m,2H),7.60 (dt,1H,J= 6.9 Hz,1.5 Hz),7.53 (dt,1H,J= 6.9 Hz,1.5 Hz),7.45 (dd, 1H,J= 8.4 Hz,7.2 Hz),7.43-7.35 (m,3H); 13C NMR (100 MHz, CDCl3):δ 133.3,133.2,131.7,130.4,128.8,128.4,128.3,128.2,126.8, 126.4,126.2,125.3,123.4,120.9,94.3,87.5; EI-MS (m/z): 228 (M+ ).
1-Phenyl-1-octyne (3am)[36]. Colorless oil. 1H NMR (400 MHz, CDCl3): δ 7.41-7.35 (m,2H),7.31-7.24 (m,3H),2.40 (t,2H, J= 7.04 Hz),1.66-1.52 (m,2H),1.50-1.38 (m,2H),1.38-1.26 (m, 4H),0.91 (t,3H,J= 6.53 Hz). 13C NMR (100 MHz,CDCl3):δ 131.6, 128.3,127.5,124.2,90.6,80.6,31.5,28.8,28.7,22.7,19.5,14.2; EIMS (m/z): 186 (M+ ). 3. Results and discussion
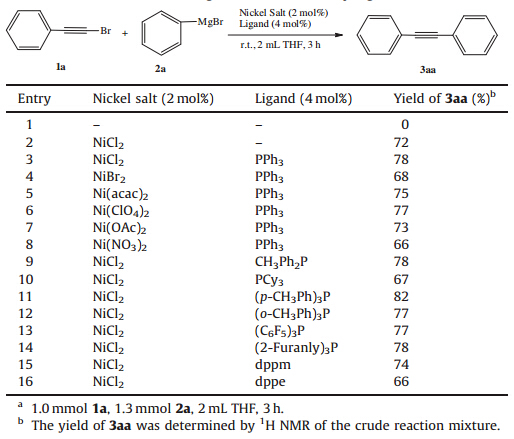
Our initial studies used (bromoethynyl)benzene (1a) and phenyl magnesium bromide (2a) as model substrates. Treatment of 1a with the Grignard reagent (2a) in THF at ambient temperature in the absence of metal and ligand did not give the 1,2-diphenylethyne (3aa) (Table 1,entry 1). When NiCl2 was used as the catalyst,the reaction of1awith the Grignard reagent (2a) produced the substituted alkyne (3aa) with a 72% conversion (Table 1,entry 2). To our delight,when 2 mol% PPh3 was used as the ligand,the NiCl2-catalyzed coupling of the substrates produced the product 3aa in 78% conversion (Table 1,entry 3). To further understand the nature of this catalysis,we tested the reaction of 1a with 2a under various conditions and the results are listed in Tables 1 and 2. Thus,different nickel precursors such as NiBr2, Ni(acac)2,Ni(ClO4)2,Ni(OAc)2 and Ni(NO3)2 were found effective to catalyze the coupling (Table 1,entries 4-8). The catalyst of NiCl2/ PPh3 complex showed the highest capability of conversion (78%) among metals/PPh3 combinations (Table 1,entry 3). The effect of ligand in the generation of 3aa using NiCl2 as the catalyst was investigated as shown in Table 1 (entries 9-16). The highest conversion was obtained when (p-CH3Ph)3P was used (Table 1, entry 11). Other ligands such as PPh3 ,PCy3,(C6F5)3P,CH3Ph2P,(oCH3Ph)3P,(2-furanyl)3P,dppm and dppe were less effective than (p-CH3Ph)3P (Table 1,entries 3,9-16).
| Table 1 Effect of the nickel source and ligand on the cross-coupling reaction.a |
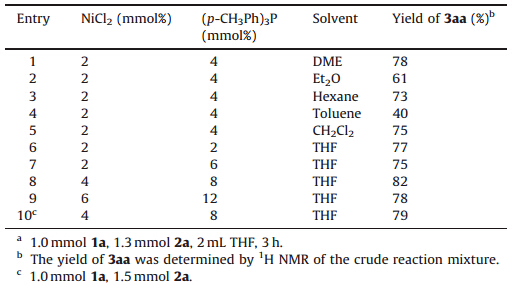
A brief examination of the influence of solvent on the yield of the product 3aa revealed that THF was the solvent of choice. In toluene,hexane,CH2Cl2,DME or Et2O,the conversion was less efficient (Table 2,entries 1-5). The molar ratio of metal and ligand was examined. It was found that a NiCl2/(p-CH3Ph)3P ratio of 1.0/ 2.0 gave the product 3aa in an optimal conversion of 82% (Table 2, entry 8). Further studies indicated that the catalyst loading dramatically influenced the conversion of the product 3aa. It was found that the most favorable catalyst loading is 4 mol% NiCl2/ 8 mol% (p-CH3Ph)3P (Table 2,entry 8). However,when the amount of the 2a was increased,the conversion of the product 3aa was decreased (Table 2,entry 10). Extensive screening showed that the optimized coupling conditions were 4 mol% NiCl2/8 mol% (pCH3Ph)3P,1.0 mmol 1a,1.3 mmol 2a in THF at room temperature (Table 2,entry 8).
| Table 2 Effect of the solvent and the molar ratio of NiCl2/(p-CH3Ph)3P on the cross-coupling reaction.a |
With the optimized conditions in hand,the scope of this reaction was studied using various Grignard reagents. The alkynyl halide 1a reacted smoothly with a series of aryl Grignard reagents 2(a-m) at room temperature to give the corresponding products 3 (aa-am) in moderate to good yields (Table 3,entries 1-13). As listed in Table 3,even with sterically hindered Grignard reagents (Table 3,entries 2,7,12),the coupling reaction underwent smoothly to give the products in moderate yields. The reaction was not significantly affected by the substituents on the aromatic ring of the Grignard reagent. Both electron-rich (Table 3,entries 2-7) and electron-deficient substituents (Table 3,entries 8-10) were tolerated. Notably,alkoxy,methyl,chloro,fluoro,trifluoromethyl, and trimethylsilanyl groups posed no challenges under the described reaction conditions. The reaction also worked well with the alkyl Grignard reagents and gave moderate yields (Table 3, entry 13).
| Table 3 NiCl2/(p-CH3Ph)3P-catalyzed cross-coupling reaction of (bromoethynyl)benzene (1a) with Grignard reagent.a |
Based on established nickel chemistry,we propose a mechanism to account for the formation of products 3(Fig. 1). The first step is the oxidative addition of (bromoethynyl)benzene (1a)to Ni(0) phosphine complex (5) (which in turn from NiCl2 and Grignard reagents) to form the organonickel(Ⅱ) bromide intermediate (6). Transmetalation of aryl magnesium bromide with 6 gives aryl-(alkenyl)nickel(Ⅱ) intermediate (7) and MgBrX. Finally, complex 7 undergoes reductive elimination to afford the desired product 3 and regenerate the active Ni(0) species (5) for the next catalytic cycle.

|
Download:
|
| Fig. 1.Proposed reaction mechanism. | |
We have developed an improved procedure for the nickelcatalyzed cross-couplings of (bromoethynyl)benzene with Grignard reagents and demonstrated that this methodology is a simple and efficient method for the preparation of 1,2-disubstituted acetylenes. In the presence of NiCl2 (4 mol%)/8 mol% (pCH2Ph)2P,(bromoethynyl)benzene reacts smoothly with 1.3 equiv. of aryl or alkyl Grignard reagents in THF at room temperature to generate the corresponding cross-coupled products in moderate to good yields. This coupling reaction is compatible with a wide range of functional groups. Notably,no other co-catalysts are necessary in the present procedure. Further applications of these 1,2-disubstituted acetylenes in organic synthesis are being investigated.
AcknowledgmentsThis work was financially supported by the Fundamental Research Funds for the Central Universities,Southwest University for Nationalities (No. 12NZYTH03),and the Natural Science Foundation of Southwest University for Nationalities (No. 381010),and the Project of Postgraduate Degree Construction, Southwest University for Nationalities (No. 2013XWD-S0703), and the State Administration of Foreign Experts Affairs project (No. 2012-10).
| [1] | J.B. Johnson, T. Rovis, More than bystanders: the effect of olefins on transitionmetal-catalyzed cross-coupling reactions, Angew. Chem. Int. Ed. 47 (2008) 840-871. |
| [2] | D.G. Yu, Z.J. Shi, Mutual activation: Suzuki-Miyaura coupling through direct cleavage of the sp2 C-O bond of naphtholate, Angew. Chem. Int. Ed. 50 (2011) 7079-7100. |
| [3] | J.J. Hirner, S.A. Blum, Nickel-catalyzed cross-coupling of organogold reagents, Organometallics 30 (2011) 1299-1302. |
| [4] | M.A. Greene, I.M. Yonova, F.J. Williams, E.R. Jarvo, Traceless directing group for stereospecific nickel-catalyzed alkyl-alkyl cross-coupling reactions, Org. Lett. 14 (2012) 4293-4296. |
| [5] | X.Q. Zhang, Z.X. Wang, Amido pincer nickel catalyzed Kumada cross-coupling of aryl, heteroaryl, and vinyl chlorides, Synlett 24 (2013) 2081-2084. |
| [6] | D.A. Everson, J.A. Buonomo, D.J. Weix, Nickel-catalyzed cross-electrophile coupling of 2-chloropyridines with alkyl bromides, Synlett 25 (2014) 233-238. |
| [7] | K. Sonogashira, Y. Tohda, N. Hagihara, A convenient synthesis of acetylenes: catalytic substitutions of acetylenic hydrogen with bromoalkenes, iodoarenes and bromopyridines, Tetrahedron Lett. 50 (1975) 4467-4470. |
| [8] | R. Chinchilla, C. Nájera, The Sonogashira reaction: a booming methodology in synthetic organic chemistry, Chem. Rev. 107 (2007) 874-922. |
| [9] | E.M. Beccalli, G. Broggini, M. Martinelli, S. Sottocornola, C-C, C-O, C-N bond formation on sp2 carbon by Pd(II)-catalyzed reactions involving oxidant agents, Chem. Rev. 107 (2007) 5318-5365. |
| [10] | D. Doucet, J.C. Hierso, Palladium-based catalytic systems for the synthesis of conjugated enynes by Sonogashira reactions and related alkynylations, Angew. Chem. Int. Ed. 46 (2007) 834-871. |
| [11] | H. Plenio, Catalysts for the Sonogashira coupling -the crownless again shall be king, Angew. Chem. Int. Ed. 47 (2008) 6954-6956. |
| [12] | M.M. Heravi, S. Sadjadi, Recent advances in the application of the Sonogashira method in the synthesis of heterocyclic compounds, Tetrahedron 65 (2009) 7761-7775. |
| [13] | K.C. Nicolaou, W.M. Dai, Chemistry and biology of the enediyne anticancer antibiotics, Angew. Chem. Int. Ed. 30 (1991) 1387-1416. |
| [14] | J.W. Grissom, G.U. Gunawardena, D. Klingberg, D.H. Huang, The chemistry of enediynes, enyne allenes and related compounds, Tetrahedron 52 (1996) 6453-6518. |
| [15] | T. Haro, C. Nevado, Gold-catalyzed ethynylation of arenes, J. Am. Chem. Soc. 132 (2010) 1512-1513. |
| [16] | B. Panda, T.K. Sarkar, On the catalytic duo PdCl2(PPh3)2/AuCl(PPh3) that cannot effect a Sonogashira-type reaction: a correction, Tetrahedron Lett. 51 (2010) 301-305. |
| [17] | M. Carril, A. Correa, C. Bolm, Iron-catalyzed Sonogashira reactions, Angew. Chem. Int. Ed. 47 (2008) 4862-4865. |
| [18] | L. Brandsma, S.F. Vasilevsky, H.D. Verkruijsse, Application of Transition Metal Catalysts in Organic Synthesis, Springer-Verlag, Berlin, 1988, pp. 179-225. |
| [19] | K.C. Nicolau, E.J. Sorensen, Classics in Total Synthesis, Wiley-VCH, Weinheim, 1996, pp. 582-586. |
| [20] | U.H.F. Bunz, Poly(aryleneethynylene)s: syntheses, properties, structures, and applications, Chem. Rev. 100 (2000) 1605-1644. |
| [21] | L.M. Tan, Z.Y. Sem, W.Y. Chong, et al., Continuous flow Sonogashira C-C coupling using a heterogeneous palladium-copper dual reactor, Org. Lett. 15 (2013) 65-67. |
| [22] | R. Ciriminna, V. Pandarus, G. Gingras, et al., Heterogeneous Sonogashira coupling over nanostructured SiliaCat Pd(0), ACS Sustainable Chem. Eng. 1 (2013) 57-61. |
| [23] | D.S. Yang, B. Li, H.J. Yang, H. Fu, L.I. Hu, Efficient copper-catalyzed Sonogashira couplings of aryl halides with terminal alkynes in water, Synlett 5 (2011) 702-706. |
| [24] | T. Suzuka, Y. Okada, K. Ooshiro, Y. Uozumi, Copper-free Sonogashira coupling in water with an amphiphilic resin-supported palladium complex, Tetrahedron 66 (2010) 1064-1069. |
| [25] | R. Severin, J. Reimer, S. Doye, One-pot procedure for the synthesis of unsymmetrical diarylalkynes, J. Org. Chem. 75 (2010) 3518-3521. |
| [26] | Y.S. Feng, Z.Q. Xu, L. Mao, F.F. Zhang, H.J. Xu, Copper catalyzed decarboxylative alkynylation of quaternary α-cyano acetate salts, Org. Lett. 15 (2013) 1472-1475. |
| [27] | S.H. Wang, L. Yu, P.H. Li, L.G. Meng, L. Wang, Copper(I) iodide catalyzed crosscoupling reaction of terminal alkynes with 1-bromoalkynes: a simple synthesis of unsymmetrical buta-1,3-diynes, Synthesis 10 (2011) 1541-1546. |
| [28] | K. Tsuyoshi, M. Nato, H. Koji, S. Tetsuya, M. Masahiro, Room temperature direct alkynylation of 1,3,4-oxadiazoles with alkynyl bromides under copper catalysis, J. Org. Chem. 75 (2010) 1764-1766. |
| [29] | M. Nato, H. Koji, S. Tetsuya, M. Masahiro, Nickel-catalyzed direct alkynylation of azoles with alkynyl bromides, Org. Lett. 11 (2009) 4156-4159. |
| [30] | J. Breitenfeld, J. Ruiz, M.D. Wodrich, X.L. Hu, Bimetallic oxidative addition involving radical intermediates in nickel-catalyzed alkyl-alkyl Kumada coupling reactions, J. Am. Chem. Soc. 135 (2013) 12004-12012. |
| [31] | Q.H. Li, H.M. Gau, Synthesis of allenes via nickel-catalyzed cross-coupling reaction of propargylic bromide with Grignard reagent, Synlett 23 (2012) 747-750. |
| [32] | A.M. Lauer, F. Mahmud, J.M. Wu, Cu(I)-catalyzed, α-selective, allylic alkylation reactions between phosphorothioate esters and organomagnesium reagents, J. Am. Chem. Soc. 133 (2011) 9119-9123. |
| [33] | G. Cahiez, O. Gager, J. Buendia, Copper-catalyzed cross-coupling of alkyl and aryl Grignard reagents with alkynyl halides, Angew. Chem. Int. Ed. 49 (2010) 1278-1281. |
| [34] | D. Castagnolo, M. Botta, Iron-Catalyzed Cross-Coupling between 1-Bromoalkynes and Grignard-Derived Organocuprate Reagents, Eur. J.Org.Chem. 17 (2010) 3224-3228. |
| [35] | H. Hofmeister, K. Annen, H. Laurent, R. Wiechert, A novel entry to 17a-bromoand 17a-iodoethynyl steroids, Angew. Chem. Int. Ed. Engl. 23 (1984) 727-729. |
| [36] | A.D. Finke, E.C. Elleby, M.J. Boyd, H. Weissman, J.S. Moore, Zinc chloride-promoted aryl bromide-alkyne cross-coupling reactions at room temperature, J. Org. Chem. 74 (2009) 8897-8900. |
| [37] | J. Moon, M. Jeong, H. Nam, et al., One-pot synthesis of diarylalkynes using palladium-catalyzed Sonogashira reaction and decarboxylative coupling of sp carbon and sp2 carbon, Org. Lett. 10 (2008) 945-948. |
| [38] | X.F. Wu, H. Neumann, M. Beller, Palladium-catalyzed Sonogashira reactions of aryl amines with alkynes via in situ formation of arenediazonium salts, Chem. Commun. 47 (2011) 7959-7961. |