b Department of Organic Chemistry, Faculty of Chemistry, University of Mazandaran, Babolsar, Iran
Quinoxaline derivatives are significant for their pharmacological activities. In particular,they exhibit potential antiviral, antibacterial,anti-inflammatory,antiprotozoal,and kinase inhibitory properties [1, 2, 3, 4, 5]. They are also utilized as dyes,electroluminescent materials,organic semiconductors,cavitands,chemically controllable switches,and DNA cleaving agents [6, 7, 8, 9, 10, 11]. Furthermore, the quinoxaline ring is the core moiety in several drug molecules,such as clofazimine,echinomycin,leromycin,and actinomycin [12, 13, 14, 15, 16, 17]. In light of the great importance of quinoxaline derivatives,in recent years efforts have been made in developing new methodologies for the synthesis of these compounds [18]. Among them,the condensation of aryl 1,2- diamines with 1,2-dicarbonyl compounds in refluxing ethanol or acetic acid is a general approach [12]. In recent years,several new efficient methods have been developed including the use of bcyclodextrin (β-CD) [19],ionic liquids [20],citric acid [21], heteropolyacid [22],cellulose sulfuric acid [23],PEG-400 [24], hypervalent iodine(III) sulfonate in PEG [25],polyaniline-sulfate salt [26],DABCO [27],CAN [28],HClO4-SiO2 [29],MnO2 [30], fluorinated alcohols [31],and (NH4)6Mo7O24·4H2O [32]. However, the reported methods still have drawbacks such as tedious workup procedures,harsh reaction conditions,low yields,long reaction times,and the requirement for an inert atmosphere. Therefore,the development of simple,efficient,high-yielding,and eco-friendly methods for quinoxaline synthesis remains an attractive goal. Recently,the use of triflic acid immobilized inorganic materials has also attracted attention,but they are all subject to leaching [33, 34]. Recent applications of polyvinylpolypyrrolidone as a reagent support [35, 36, 37, 38] have been extensively investigated. We have recently demonstrated that polyvinylpolypyrrolidoniume triflate efficiently catalyzed reaction between indole and aldehydes for the preparation of bis-indolyl methane derivatives [39]. In continuing our studies on the application of new reagents or systems for organic functional group transformations [40, 41, 42],we report a new application of polyvinylpolypyrrolidoniume triflate as an efficient, mild,noncorrosive,and recyclable catalyst in an alternative method for the synthesis of quinoxaline derivatives (Scheme 1).

|
Download:
|
| Scheme 1.Synthesis of 2,3-disubstituted quinoxalines derivatives in water. | |
To a suspension of polyvinylpolypyrrolidone (3.0 g) in toluene (35 mL),TfOH (2.0 g,13 mmol) was added. The mixture was stirred magnetically for 60 min at r.t. The toluene was removed under reduced pressure and the residue was dried at 110 ℃ for 2 h to afford PVPP·OTf as a white powder. The number of H+ sites in PVPP·OTf as determined by acid-base titration was 10 mmol g-1. 2.2. Typical experimental procedure
A mixture of 1,2-dicarbonyl compounds (1 mmol),aryl 1,2- diamines (1 mmol) dissolved in 4 mL water,and PVPP·OTf (30 mg) was stirred for 1 h. The reaction was monitored by TLC. After completion of the reaction,the mixture was washed with chloroform and filtered to recover the catalyst. The filtrate was evaporated and purified by recrystallization from hot ethanol to afford pure products. Products were characterized by comparison of their physical and spectral data with those of authentic samples. Spectroscopic data for selected examples as follows:
2,3-Diphenylquinoxaline (Table 1,entry 1): White solid; mp 126-127 ℃; IR (KBr,cm-1): n 3051,1630,1528,1348,772; 1H NMR (400 MHz,CDCl3): δ 7.33-7.41 (m,6H),7.53-7.57 (m,4H), 7.76-7.83 (m,2H),8.20-8.23 (m,2H); 13C NMR (100 MHz,CDCl3): δ 128.2,128.9,129.2,129.8,129.9,139.1,141.2,153.4.
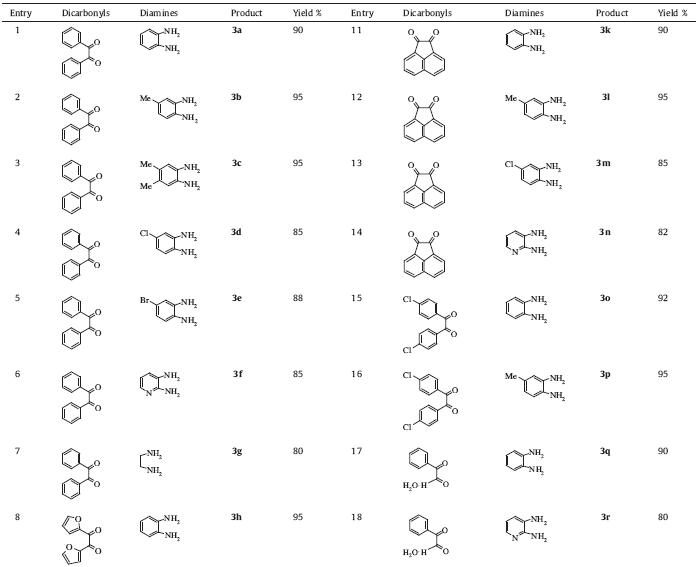
| Table 1 Quinoxaline derivatives from the reaction of 1,2-diamines and 1,2-diketones catalyzed by PVPP·OTf. |
2,3-Diphenylpyrido[2,3,b]pyrazine (Table 1,entry 6): Yellow crystals; mp 135-137 ℃; IR (KBr,cm-1): n 3055,1640,1530,1340; 1H NMR (400 MHz,CDCl3): δ 7.34-7.45 (m,5H),7.59-7.66 (m,4H), 7.73-7.76 (m,2H),8.54-8.56 (m,1H),9.20 (δ,1H,J = 4.8 Hz); 13C NMR (100 MHz,CDCl3): δ 125.6,128.5,128.8,129.7,129.8, 130.2,130.6,136.6,138.5,138.9,150.2,154.4,155.1,156.7.
Acenaphtho[1,2,b]quinoxaline (Table 1,entry 11): White solid; mp 242-245 ℃. IR (KBr,cm-1): n 3443,3047,2922,2361,1614, 1481. 1H NMR (400 MHz,CDCl3): δ 7.70-7.75 (m,2H),7.98 (t,2H, J = 7.7 Hz),8.15 (δ,2H,J = 7.7 Hz),8.18-8.22 (m,2H),8.42 (δ,2H, J = 7.7 Hz); 13C NMR (100 MHz,CDCl3): δ 125.7,126.8,127.2,128.2, 128.9,129.6,130.1,133.2,142.5,145.3. 3. Results and discussion
To initiate our study,the reaction of benzil (1 mmol) with ophenylenediamine was chosen as a model reaction in water at room temperature. The corresponding 2,3-disubstituted quinoxalines 3a was obtained in high yield (90%) after 60 min (Table 1, entry 1). The new methodology allowed us to prepare the 2,3- disubstituted quinoxalines shown in Table 1.
In order to evaluate the efficiency of this methodology,various arene-1,2-diamines,such as mono- and disubstituted amines, reacted efficiently with 1,2-dicarbonyl compounds to give the corresponding 2,3-disubstituted quinoxalines (Table 1). Results in Table 1 show that electron-donating groups at the phenyl ring of 1,2-diamine favoured the formation of product (Table 1,entries 1 and 2) In contrast,electron-withdrawing groups such as chloro and bromo gave slightly lower yields (85%-88%) (Table 1,entries 4 and 5). Interestingly,2,3-diaminopyridine underwent smooth coupling with benzil to afford the corresponding pyrido[2,3,b]pyrazine 3f in 85% yield (Table 1,entry 6). Similarly,several 1,2-dicarbonyl compounds,such as furil,1,2-di(4-chlorophenyl)ethanedione, phenylglyoxal,ninhydrin,and acenaphthene-1,2-quinone also reacted rapidly with 1,2-diamines to produce a variety of quinoxaline derivatives (Table 1,entries 8-19). In all cases,the reactions proceeded rapidly at room temperature with high efficiency. The products were characterized by 1H NMR and 13C NMR,IR spectroscopic data,and also by comparison with authentic samples. This method not only affords the products in excellent yields but also avoids the problems associated with catalyst cost, handling,safety,and pollution. One of the major advantages of this protocol is the isolation and purification of the products,which have been achieved by simple filtration and crystallization of the crude products. The characterization of the Brønsted acid sites present in the polymer was performed by recording the FT-IR spectrum of PVPP·OTf,which shows a strong broad absorption at 3400 cm-1 for the O-H bond and a moderate absorption at 1648 cm-1 that corresponds to the internal imine groups present in the pendant rings of the polymer (Fig. 1). Respectively,the bands at 1225 cm-1 and 1174 cm-1 were assigned to the S55O asymmetric and symmetric stretching vibrations of -SO3- group [37]. Loading capacity of the reagent was determined by titration and found to be 10 mmol g-1,whereas its silica supported analogue has a loading capacity of less than 1 mmol g-1.

|
Download:
|
| Fig. 1.The FT-IR spectrum of polyvinylpolypyrrolidone (PVPP) and (PVPP·OTf) catalyst. | |
As PVPP·OTf is not soluble in acetonitrile, no PVPP·OTf leaching as well as no contribution of homogeneous catalysis in the course of reaction was expected. To prove this, after 1 h, the catalyst was removed from acetonitrile by filtration and the supernatant was tested for activity. No activity was observed, indicating that there was no contribution of homogeneous catalysis in this reaction. After reaction, the catalyst can be easily separated (by filtration) and reused without decrease in its activity. For example, the reaction of benzil and o-phenylenediamine afforded the corresponding 2,3-diphenylquinoxaline derivatives in 90%, 90%, 88%, 88%, and 85% isolated yields over five cycles. We believe that the procedure is simple and convenient, and it does not require any aqueous work-up, thereby avoiding the generation of waste, and so it may contribute to the area of green chemistry. 4. Conclusion
In conclusion, we have developed a simple and highly efficient method for the synthesis of quinoxaline derivatives through the reaction of aryl 1,2-diamines with 1,2-dicarbonyl compounds at room temperature catalyzed by polyvinylpolypyrrolidoniume triflate in water. The advantages of this procedure are operational simplicity, wide substrate scope, and high yields. Acknowledgment
This research is supported by the Islamic Azad University, Ayatollah Amoli Branch.
| [1] | C.W. Lindsley, Z. Zhao, W.H. Leister, et al., Allosteric Akt (PKB) inhibitors: discovery and SAR of isozyme selective inhibitors, Bioorg. Med. Chem. Lett. 15 (2005) 761-764. |
| [2] | M. Loriga, S. Piras, P. Sanna, et al., Quinoxaline chemistry. Part 7. 2-[Aminobenzoates]-and 2-[aminobenzoylglutamate]-quinoxalines as classical antifolate agents. Synthesis and evaluation of in vitro anticancer, anti-HIV and antifungal activity, Farmaco 52 (1997) 157-166. |
| [3] | L.E. Seitz, W.J. Suling, R.C. Reynolds, Synthesis and antimycobacterial activity of pyrazine and quinoxaline derivatives, J. Med. Chem. 45 (2002) 5604-5606. |
| [4] | W. He, M.R. Meyers, B. Hanney, et al., Potent quinoxaline-based inhibitors of PDGF receptor tyrosine kinase activity. Part 2. The synthesis and biological activities of RPR127963 an orally bioavailable inhibitor, Bioorg. Med. Chem. Lett. 13 (2003) 3097-3100. |
| [5] | Y.B. Kim, Y.H. Kim, J.Y. Park, et al., Synthesis and biological activity of new quinoxaline antibiotics of echinomycin analogues, Bioorg. Med. Chem. Lett. 14 (2004) 541-544. |
| [6] | A. Katoh, T. Yoshida, J. Ohkanda, Synthesis of quinoxaline derivatives bearing the styryl and phenylethynyl groups and application to a fluorescence derivatization reagent, Heterocycles 52 (2000) 911-920. |
| [7] | K.R.J. Thomas, M. Velusamy, J.T. Lin, C.H. Chuen, Y.T. Tao, Chromophore-labeled quinoxaline derivatives as efficient electroluminescent materials, Chem. Mater. 17 (2005) 1860-1866. |
| [8] | S. Dailey, W.J. Feast, R.J. Peace, et al., Synthesis and device characterisation of sidechain polymer electron transport materials for organic semiconductor applications, J. Mater. Chem. 11 (2001) 2238-2243. |
| [9] | J.L. Sessler, H. Maeda, T. Mizuno, et al., Quinoxaline-bridged porphyrinoids, J. Am. Chem. Soc. 124 (2002) 13474-13479. |
| [10] | M.J. Crossley, L.A. Johnston, Laterally-extended porphyrin systems incorporating a switchable unit, Chem. Commun. (2002) 1122-1123. |
| [11] | T. Yamaguchi, S. Matsumoto, K. Watanabe, Generation of free radicals from dihydropyranzines with DNA strand-breakage activity, Tetrahedron Lett. 39 (1998) 8311. |
| [12] | J.D. Brown, The chemistry of heterocyclic compounds, quinoxalines, in: C.E. Taylor, P. Wipf (Eds.), Supplements II, John Wiley and Sons, New Jersey, 2004. |
| [13] | R.S. Bhosale, S.R. Sarda, S.S. Andhapure, et al., An efficient protocol for the synthesis of quinoxaline derivatives at room temperature using molecular iodine as the catalyst, Tetrahedron Lett. 46 (2005) 7183-7186. |
| [14] | S.V. More, M.N.V. Sastry, C.C. Wang, et al., Molecular iodine: a powerful catalyst for the easy and efficient synthesis of quinoxalines, Tetrahedron Lett. 46 (2005) 6345-6348. |
| [15] | Y.S. Beheshtiha, M.M. Heravi, M. Saeedi, et al., Efficient and green synthesis of 1,2-disubstituted benzimidazoles and quinoxalines using Brønsted acid ionic liquid,[(CH2)4SO3HMIM][HSO4], in water at room temperature, Synth. Commun. 40 (2010) 1216-1223. |
| [16] | T.M. Potewar, S.A. Ingale, K.V. Srinivasan, Efficient synthesis of quinoxalines in the ionic liquid 1-n-butylimidazolium tetrafluoroborate ([Hbim]BF4) at ambient temperature, Synth. Commun. 38 (2008) 3601-3612. |
| [17] | D. Fang, K. Gong, Z.H. Fei, X.L. Zhou, Z.L. Liu, A practical and efficient synthesis of quinoxaline derivatives catalyzed by task-specific ionic liquid, Catal. Commun. 9 (2008) 317. |
| [18] | A.E.A. Porter, A.R. Katritsky, Comprehensive Heterocyclic Chemistry, Pergamon, Oxford, 1984, pp. 157-197. |
| [19] | B. Madhav, S. Narayana Murthy, V. Prakash Reddy, et al., Biomimetic synthesis of quinoxalines in water, Tetrahedron Lett. 50 (2009) 6025-6028. |
| [20] | H.M. Meshram, P. Ramesh, G. Santosh Kumar, et al., One-pot synthesis of quinoxaline-2-carboxylate derivatives using ionic liquid as reusable reaction media, Tetrahedron Lett. 51 (2010) 4313-4316. |
| [21] | R. Mahesh, A.K. Dhar, T.T.V.N.V. Sasank, et al., Citric acid: an efficient and green catalyst for rapid one pot synthesis of quinoxaline derivatives at room temperature, Chin. Chem. Lett. 22 (2011) 389-392. |
| [22] | T.K. Huang, L. Shi, R. Wang, et al., Keggin type heteropolyacids-catalyzed synthesis of quinoxaline derivatives in water, Chin. Chem. Lett. 20 (2009) 161-164. |
| [23] | A. Shaabani, A.H. Rezayan, M. Behnam, et al., Green chemistry approaches for the synthesis of quinoxaline derivatives: Comparison of ethanol and water in the presence of the reusable catalyst cellulose sulfuric acid, C. R. Chim. 12 (2009) 1249-1252. |
| [24] | X.Z. Zhang, J.X. Wang, Y.J. Sun, H.W. Zhan, Synthesis of quinoxaline derivatives catalyzed by PEG-400, Chin. Chem. Lett. 21 (2010) 395-398. |
| [25] | P.Y. Lin, R.S. Hou, H.M. Wang, L.J. Kang, L.C. Chen, Hypervalent Iodine(III) sulfonate mediated synthesis of quinoxalines in liquid PEG-400, J. Chin. Chem. Soc. 56 (2009) 683-687. |
| [26] | C. Srinivas, C.N.S.S.P. Kumar, V.J. Rao, et al., Efficient, convenient and reusable polyaniline-sulfate salt catalyst for the synthesis of quinoxaline derivatives, J. Mol. Catal. A: Chem. 265 (2007) 227-230. |
| [27] | H.M. Meshram, G. Santosh Kumar, P. Ramesh, et al., A mild and convenient synthesis of quinoxalines via cyclization-oxidation process using DABCO as catalyst, Tetrahedron Lett. 51 (2010) 2580-2585. |
| [28] | S.V. More, M.N.V. Sastry, C.F. Yao, Cerium (IV) ammonium nitrate (CAN) as a catalyst in tap water: a simple, proficient and green approach for the synthesis of quinoxalines, Green Chem. 8 (2006) 91-95. |
| [29] | B. Das, K. Venkateswarlu, K. Sunnel, A. Majhi, An efficient and convenient protocol for the synthesis of quinoxalines and dihydropyrazines via cyclization-oxidation processes using HClO4·SiO2 as a heterogeneous recyclable catalyst, Tetrahedron Lett. 48 (2007) 5371-5374. |
| [30] | S.Y. Kim, K.H. Park, Y.K. Chung, Manganese(IV) dioxide-catalyzed synthesis of quinoxalines under microwave irradiation, Chem. Commun. (2005) 1321-1323. |
| [31] | S. Khaksar, F. Rostamnezhad, A novel one-pot synthesis of quinoxaline derivatives in fluorinated alcohols, Bull. Korean Chem. Soc. 33 (2012) 2581-2584. |
| [32] | A. Hasaninejada, A. Zareb, M.R. Mohammadizadeha, Z. Karami, Synthesis of quinoxaline derivatives via condensation of aryl-1,2-diamines with 1,2-diketones using (N4)6Mo7O24. 4H2O as an efficient, mild and reusable catalyst, J. Iran. Chem. Soc. 1 (2009) 153-158. |
| [33] | P.N. Liu, F. Xia, Q.W. Wang, Y.J. Ren, J.Q. Chen, Triflic acid adsorbed on silica gel as an efficient and recyclable catalyst for the addition of β-dicarbonyl compounds to alcohols and alkenes, Green Chem. 12 (2010) 1049-1055. |
| [34] | A.D. Angelis, C. Flego, P. Ingallina, et al., Studies on supported triflic acid in alkylation, Catal. Today 65 (2001) 363-371. |
| [35] | M.M. Lakouraj, F. Najafizadeh, Polyvinylpolypyrrolidone-bound boron trifluoride (PVPP-BF3); a mild and efficient catalyst for synthesis of 4-metyl coumarins via the Pechmann reaction, C. R. Chim. 15 (2012) 530-532. |
| [36] | M.M. Lakouraj, M. Mokhtary, Polyvinylpolypyrrolidone-bromine complex: mild and efficient polymeric reagent for bromination of activated aromatic compounds, Chin. Chem. Lett. 22 (2011) 13-17. |
| [37] | M.M. Lakouraj, M. Mokhtary, Polyvinylpolypyrrolidone-bromine complex, mild and efficient polymeric reagent for selective deprotection and oxidative deprotection of silyl ethers, Lett. Org. Chem. 4 (2007) 64-67. |
| [38] | A. Ghorbani-Choghamarani, G. Azadi, Polyvinylpolypyrrolidone-supported hydrogen peroxide (PVP-H2O2), silica sulfuric acid and catalytic amounts of ammonium bromide as green, mild and metal-free oxidizing media for the efficient oxidation of alcohols and sulfides, J. Iran. Chem. Soc. 4 (2011) 1082-1090. |
| [39] | S. Khaksar, M. Tajbakhsh, M. Gholami, Polyvinylpolypyrrolidone-supported triflic acid (PVPP-OTf) as a new, efficient, and recyclable heterogeneous catalyst for the synthesis of bis-indolyl methane derivatives, C. R. Chim. 17 (2014) 30-34. |
| [40] | S. Khaksar, E. Fattahi, E. Fattahi, Organocatalytic synthesis of amides from nitriles via the Ritter reaction, Tetrahedron Lett. 52 (2011) 5943-5946. |
| [41] | S. Khaksar, S.M. Talesh, Three-component one-pot synthesis of 2,3-dihydroquinazolin-4(1H)-one derivatives in 2,2,2-trifluoroethanol, C. R. Chim. 15 (2012) 779-783. |
| [42] | S. Khaksar, S.M. Vahdat, R.N. Moghaddamnejad, Pentafluorophenylammonium triflate: an efficient, practical, and cost-effective organocatalyst for the Biginelli reaction, Monatsh Chem. 143 (2012) 1671-1674. |