b Institute of Chemical Sciences, Organic Chemistry Division, Bahauddin Zakariya University, Multan 60800, Pakistan;
c Department of Chemistry, Faculty of Engineering, Gifu University, Yanagido, Gifu 501-1193, Japan
The assembly of complex polycyclic skeletons of chemical and biomedical interest has become an important,challenging and active area of research in modern organic chemistry [1]. Among these skeletons,isocoumarin based fused ring system present in many natural products (Fig. 1) shows a broad range of biological activities [2]. In recent years,a variety of methods have been developed to prepare these structurally complex fused skeletons [3]. However,synthetic chemists are continuously searching for the development of new,cleaner and efficient chemical transformation methodologies,or modifications in the established synthetic pathways to ensure eco-friendly and cost effective synthesis with minimal or no use of toxic chemicals.

|
Download:
|
| Fig. 1. Some representative natural products. | |
Ti ll date,excellent region-,chemo-,diastereo- and enantioselectivities are obtained for the preparation of complex molecules by developing several highly selective procedures [4]. The procedure usually used for the construction of such organic compounds involves a step-wise formation of individual bonds in the target molecule. However,it is much more desirable,if one could form several bonds in one go without isolating the intermediates and changing the reaction conditions. The waste produced in such synthetic procedures is very small as compared to step-wise pathways. Therefore,from the synthetic point of view, one-pot synthesis of fused-ring systems is an attractive procedure for searching bioactive compounds.
Coumarin derivatives [5] are important synthetic targets because of possessing diverse biological applications [6]. They are also known to have vasodilatory [7],anticoagulant [8],anti-HIV [9],antitumor [10] and anti-inflammatory [11] properties. The fluorescent properties of some isocoumarin derivatives are also reported [12]. Herein,we report one-pot synthesis of novel fused isocoumarin framework through highly selective domino multicyclizations under catalyst and solvent free reaction conditions. The resulting fused isocoumarin framework is an interesting scaffold for drug design and discovery and can play an important role in pharmaceutical research. 2. Experimental 2.1. Materials and methods
All reagents and solvents were used as obtained from the supplier or recrystallized/redistilled as required. Thin layer chromatography (TLC) was performed using aluminum sheets (Merck) coated with silica gel 60 F254. The melting points of compounds were determined using capillary tubes and an electrothermal melting point apparatus,model MP-D Mitamura Riken Kogyo,Japan. IR spectra of compounds were recorded on a Bio-Rad FTS 3000 MX spectrophotometer (400-4000 cm-1 ). NMR spectra were recorded using a Bruker AM-300 spectrometer and chemical shifts are reported in ppmversustetramethylsilane with either tetramethylsilane or the residual solvent resonance used as an internal standard. Mass spectra were acquired on a Bruker Omniflex MALDI-TOF instrument and elemental analyses were carried out with a LECO-183 CHNS model. 2.2. Procedure for the synthesis of hexacyclic fused isocoumarin (6)
A mixture of 2,3-diphenylacrylic acid (2.07 g,9 mmol) and thionyl chloride (1 mL) was heated in the presence of a few drops of DMF for 30 min at 70°C. Completion of reaction was indicated by the disappearance of gas evolution. Excess thionyl chloride was removed under reduced pressure to afford 2,3-diphenylacryloyl chloride. Homophthalic acid (0.54 g,3 mmol) was then added to it and the mixture was heated first for 3.5 h at 200°C and then cooled to room temperature. Addition of aqueous solution of sodium carbonate (5%,200 mL),followed by filtration and washing thoroughly with water furnished the crude product,which was recrystallized in toluene to give compound 6 in pure form (68%). Mp: 135-136°C; IR (KBr,cm-1):v 2918 (C-H),1704 (C=O),1569 (C=C); 1H NMR (300 MHz,CDCl3):δ 8.11 (dd,1H,J= 1.2,7.5 Hz), 7.91 (d,1H,J= 7.5 Hz),7.88-7.82 (m,1H),7.59-7.19 (m,12H),6.98 (dd,2H,J= 1.2,7.5 Hz),4.96 (s,1H); 13C NMR (75 MHz,CDCl3):δ 204.6,161.4,151.1,142.7,137.5,135.8,135.7,135.3,133.1,130.6, 128.8,128.7,128.6,128.4,128.2,127.4,124.9,124.4,123.6,122.7, 107.8,62.7,48.2,31.0; MS [MALDI-TOF] (m/z): 427.13 [M+H+ ] (100),428.13 (33),429.13 (5). Anal. Calcd. for C30H18O3: C,84.49; H,4.25; Found: C,84.53; H,4.23. 3. Results and discussion
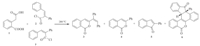
We commenced our studies by reacting equimolar quantities of homophthalic acid1and 2,3-diphenylacryloyl chloride 2 at 200°C with an aim of getting isocoumarin derivative,3-(1,2-diphenylvinyl)-1H-isochromen-1-one 3 [13] (Scheme 1 and Fig. S1 in Supporting information). However,instead of obtaining the expected product 3,compound 4,i.e. 3-phenyl-1H-isochromen-1-one [14] and the novel fused hexacyclic isocoumarin framework 6,formed by the domino multicylization reaction,were isolated in 75% and 1% yields,respectively (Table 1,entry 1). Structure of the novel fused ring system6was established through spectral (IR, 1H NMR, 13C NMR,COSY,NOESY,MS) and single crystal X-ray analyses (Fig. 2,Table 2). This unprecedented observation prompted us to pursue further the domino cyclization reaction from both the mechanistic as well as synthetic viewpoints. Accordingly,we retroanalyzed the fused isocoumarin skeleton 6 (Fig. S2 in Supporting information); it was hypothesized that some of the 2,3-diphenylacryloyl chloride 2 might be cyclized under the conditions to give 2-phenyl-1H-inden-1-one 5 [15],which reacted with the already observed 3-phenyl-1H-isochromen-1-one 4 through Michael addition reaction. To check our hypothesis,the concentration of 2 was gradually increased under the same reaction conditions. To our delight,the yield of 6significantly increased (1%→20%),while that of isocoumarin derivative 4 diminished (75%→45%) (Table 1,entry 2). Interestingly,when the concentration of2was enhanced to 3 equivalents,the yield of6 further increased (20%→30%),while that of 4 decreased (45%→28%) (Table 1,entry 3). At this stage,it was assumed that the formation of 2-phenyl-1H-inden-1-one 5compared with 3-phenyl-1H-isochromen-1-one4was slow and it needed more time to cyclize. Thus,the reaction time was increased from 2 to 3 h. It was found that the yield of4pronouncedly decreased (28%→7%), while that of 6 increased (30%→46%) (Table 1,entry 4). Surprisingly,further increase in the time duration (3→4 h) led to the formation of compounds 5 and 6 in 3% and 67% yields,respectively (Table 1,entry 5). It is pertinent to mention that compound4was not isolated under the conditions,indicating that compound 5reacts with it very quickly as soon as it forms. Interestingly,a slight decrease in the time duration (4→3.5 h) provided maximum yield (68%) of the fused compound6along with a trace amount of compound 5 as an impurity (Table 1,entry 6).
| Table 1 Optimization of reaction conditions for the synthesis of 6. |
| Table 2 X-ray crystallographic data of 6. |
|
Download:
|
| Scheme 1. Novel domino multicyclization reaction with all possible byproducts. | |
The attractive feature of this domino reaction is demonstrated by the fact that four new chemical bonds and three new rings were readily formed in domino fashion. In addition,work-up of the reaction is very simple. Water and phenylacetylene are the only byproducts,which may be evaporated under the reaction conditions/during the concentration of the reaction mixture, making the work-up very convenient simply by adding water/ filtration/washing/recrystallization. Finally,it is important to address here that only a single diastereomer of 6 was detected first by spectroscopic and then by X-ray diffraction analysis (Fig. 2 and Table 2).

|
Download:
|
| Fig. 2. ORTEP diagram of 6. | |
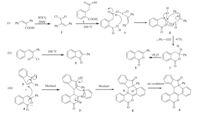
The mechanism for this domino multicyclization reaction is proposed and shown in Scheme 2. It can be divided into three steps. The first step involves ring closure cascade reaction,which consists of regioselective condensation of acyl chloride 2 with homophthalic acid1leading to A and HCl (2 to A),intramolecular cyclization (A to B),removal of CO2,phenyl acetylene and phenyl migration in a concerted fashion (BtoC),and finally dehydration (C to 4). The second step includes intramolecular cyclization of 2 to give intermediate 5. In the third step,double Michael addition reaction between 4 and 5 leads to intermediateEthroughD,which after aerobic oxidation,provides thermodynamically stable hexacyclic fused isocoumatin framework6. This mechanism has been partially supported by an experiment in which the isolated intermediates 4 and 5 were reacted at 200°C under solvent and catalyst free conditions; the hexacyclic product 6 was again generated in 66% yield (Scheme S1 in Supporting information). To the best of our knowledge,the synthetic strategy and mechanistic sequences described herein have not been reported so far.
|
Download:
|
| Scheme 2. Proposed mechanism for the synthesis of 6. | |
Conclusively,a novel three component domino reaction was used for the construction of unprecedented hexacyclic fused isocoumarin framework. It is noteworthy for its cheap and readily available starting materials,eco-friendly procedure,easy work-up and potential biological applications of the resulting product. Our future efforts will be focused on using various computational and experimental methods for exploring the biological applications of this novel fused ring system. The facile one pot synthetic procedure may also be used to construct more useful and potential bioactive derivatives of this fused isocoumarin skeleton. Acknowledgment
We are highly grateful to the Higher Education Commission (HEC),Govt. of Pakistan for financial support. Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.03.022.
| [1] | (a) G.L. Adams, P.J. Carroll, A.B. Smith, Access to the Akuammiline family of alkaloids: total synthesis of (+)-scholarisine A, J. Am. Chem. Soc. 135 (2013) 519-528; (b) W. Fan, Q. Ye, H.W. Xu, et al., Novel double [3+2+1] heteroannulation for forming unprecedented dipyrazolo-fused 2,6-naphthyridines, Org. Lett. 15 (2013) 2258-2261; (c) B. Jiang, X. Wang, H.W. Xu, et al., Highly selective domino multicyclizations for forming polycyclic fused acridines and azaheterocyclic skeletons, Org. Lett. 15 (2013) 1540-1543; (d) M. Piltan, I. Yavari, L. Moradi, Tandem synthesis of functionalized hexaalkyl benzoisoquinolinopyrrolonaphthyridine-hexacarboxylate, via isoquinoline based multi-component reaction, Chin. Chem. Lett. 24 (2013) 979-983; (e) A.D. Melhado, W.E. Brenzovich, A.D. Lackner, F.D. Toste, Gold-catalyzed threecomponent coupling: oxidative oxyarylation of alkenes, J. Am. Chem. Soc. 132 (2010) 8885-8887; (f) R.A. Yoder, J.N. Johnston, A case study in biomimetic total synthesis: polyolefin carbocyclizations to terpenes and steroids, Chem. Rev. 105 (2005) 4730-4756. |
| [2] | (a) P.C. Chao, C.C. Hsu, M.C. Yin, Anti-inflammatory and anti-coagulatory activities of caffeic acid and ellagic acid in cardiac tissue of diabetic mice, Nutr. Metab. 6 (2009) 33; (b) G. Cozza, A. Gianoncelli, P. Bonvini, et al., Urolithin as a converging scaffold linking ellagic acid and coumarin analogues: design of potent protein kinase CK2 inhibitors, ChemMedChem 6 (2011) 2273-2286; (c) H.A. De Abreu, I.A.D.S. Lago, G.P. Souza, et al., Antioxidant activity of (+)-bergenin -a phytoconstituent isolated from the bark of Sacoglottis uchi Huber (Humireaceae), Org. Biomol. Chem. 6 (2008) 2713-2718; (d) J.H. Weisburg, A.G. Schuck, S.E. Reiss, et al., Ellagic acid, a dietary polyphenol, selectively cytotoxic to HSC-2 oral carcinoma cells, Anticancer Res. 33 (2013) 1829-1836; (e) T.Y. Kao, Y.C. Chung, Y.C. Hou, et al., Effects of ellagic acid on chemosensitivity to 5-fluorouracil in colorectal carcinoma cells, Anticancer Res. 32 (2012) 4413-4418; (f) Y.S. Kim, T. Zerin, H.Y. Song, Antioxidant action of ellagic acid ameliorates paraquat-induced A549 cytotoxicity, Biol. Pharm. Bull. 36 (2013) 609-615. |
| [3] | (a) F. Nemati, R. Saeedirad, Nano-Fe3O4 encapsulated-silica particles bearing sulfonic acid groups as a magnetically separable catalyst for green and efficient synthesis of functionalized pyrimido[4,5-b]quinolines and indeno fused pyrido[ 2,3-d]pyrimidines in water, Chin. Chem. Lett. 24 (2013) 370-372; (b) W.C. Gong, Y. Liu, J. Zhang, et al., Regio-and stereoselective [4+3] cycloaddition towards fused 5,7,6-tricyclic skeletons, Chem. Asian J. 8 (2013) 546-551; (c) S. Yasuike, M. Niwa, K. Yamaguchi, T. Tsuchiya, J. Kurita, Synthesis of 1-stibaphenalenes, the first example of group 15 phenalenes, via a 1,5-dilithium intermediate, Chem. Lett. 30 (2001) 554-555; (d) J.J. Kaloko, Y.H. Gary, T.I. Ojima, One-step formation of fused tetracyclic skeletons from cyclohexene-diynes and carbon monoxide through Rh(I)-catalyzed[2+2+2+1] cycloaddition reaction, Chem. Commun. (2009) 4569-4571; (e) H. Mizoguchi, H. Oguri, K. Tsuge, H. Oikawa, Divergent and expeditious access to fused skeletons inspired by indole alkaloids and transtaganolides, Org. Lett. 11 (2009) 3016-3019; (f) N. Saito, T. Ichimaru, Y. Sato, Ruthenium-catalyzed intramolecular [2+2+2] cyclization of allene-yne-enes: construction of fused-tricyclic skeletons, Chem. Asian J. 7 (2012) 1521-1523; (g) S. Rostamizadeh, M. Nojavan, R. Aryan, H. Sadeghian, M. Davoodnejad, A novel and efficient synthesis of pyrazolo[3,4-d]pyrimidine derivatives and the study of their anti-bacterial activity, Chin. Chem. Lett. 24 (2013) 629-632; (h) F. Shi, X.N. Zeng, X.D. Cao, et al., Design and diversity-oriented synthesis of novel 1,4-thiazepan-3-ones fused with bioactive heterocyclic skeletons and evaluation of their antioxidant and cytotoxic activities, Bioorg. Med. Chem. Lett. 22 (2012) 743-746. |
| [4] | (a) A. Ahmed, S. Dhara, J.K. Ray, Palladium-catalyzed and (KOBu)-Bu-t-promoted C-aryl-O-alcoholic coupling: an efficient one-pot synthesis of oxygen containing fused rings, Tetrahedron Lett. 54 (2013) 1673-1676; (b) P. Sang, M. Yu, H.F. Tu, J.W. Zou, Y.H. Zhang, Highly regioselective synthesis of fused seven-membered rings through copper-catalyzed cross-coupling, Chem. Commun. 49 (2013) 701-703; (c) A. Caruso, M.A. Siegler, J.D. Tovar, Synthesis of functionalizable boron-containing pi-electron materials that incorporate formally aromatic fused borepin rings, Angew. Chem. Int. Ed. 49 (2010) 4213-4217; (d) M.A. Esteruelas, I. Fernandez, A. Herrera, et al., Multiple C-H bond activation of phenyl-substituted pyrimidines and triazines promoted by an osmium polyhydride: formation of osmapolycycles with three, five, and eight fused rings, Organometallics 29 (2010) 976-986; (e) D.R. Levine, A. Caruso, M.A. Siegler, J.D. Tovar, Meta-B-entacenes: new polycyclic aromatics incorporating two fused borepin rings, Chem. Commun. 48 (2012) 6256-6258; (f) S. Maiti, M.G.B. Drew, R. Mukhopadhyay, B. Achari, A.K. Banerjee, Convenient formation of six-to nine-membered carbocyclic rings by 2-pyridyl radical cyclization: a generalized synthesis of pyridine-fused linear tricyclic systems, Synthesis-Stuttgart (2005) 3067-3078; (g) K. Niimi, S. Shinamura, I. Osaka, E. Miyazaki, K. Takimiya, Dianthra[2,3-b:2030-f]thieno[3,2-b]thiophene (DATT): synthesis, characterization, and FET characteristics of new π-extended heteroarene with eight fused aromatic rings, J. Am. Chem. Soc. 133 (2011) 8732-8739; (h) V. Novakova, J. Roh, P. Gela, J. Kunes, P. Zimcik, Azaphthalocyanines with fused triazolo rings: formation of sterically stressed constitutional isomers, Chem. Commun. 48 (2012) 4326-4328; (i) L.A. Paquette, R.V.C. Carr, E. Arnold, J. Clardy, Electronic control of stereoselectivity. 5. Stereochemistry of singlet oxygen capture by cyclopentadiene rings fused to norbornyl and norbornenyl frameworks, J. Org. Chem. 45 (1980) 4907-4913. |
| [5] | (a) D. Du, Z.W. Wang, N-Heterocyclic carbene-catalyzed domino reactions of formylcyclopropane 1,1-diesters: a new synthesis of coumarins, Eur. J. Org. Chem. (2008) 4949-4954; (b) M.A. Kinder, P. Margaretha, Photochemistry of 4H,7H-benzo[1,2-c: 4,3-c0]dipyran-4,7-dione, a twofold isocoumarin, Org. Lett. 2 (2000) 4253-4255; (c) V. Nair, C.R. Sinu, R. Rejithamol, K.C.S. Lakshmi, E. Suresh, A novel NHCcatalyzed transformation of 2H-chromene-3-carboxaldehydes to 3-methyl-2Hchromen-2-ones, Org. Biomol. Chem. 9 (2011) 5511-5514; (d) S. Ozcan, M. Balci, The chemistry of homophthalic acid: a new synthetic strategy for construction of substituted isocoumarin and indole skeletons, Tetrahedron 64 (2008) 5531-5540; (e) E.M. Phillips, M. Wadamoto, H.S. Roth, A.W. Ott, K.A. Scheidt, NHC-catalyzed reactions of aryloxyacetaldehydes: a domino elimination/conjugate addition/acylation process for the synthesis of substitutedcoumarins,Org. Lett.11(2009) 105-108; (f) S.P. Waters, M.C. Kozlowski, Synthesis of the isocoumarin portion of the rubromycins, Tetrahedron Lett. 42 (2001) 3567-3570. |
| [6] | (a) F. Borges, F. Roleira, N. Milhazes, L. Santana, E. Uriarte, Simple coumarins and analogues in medicinal chemistry: occurrence, synthesis and biological activity, Curr. Med. Chem. 12 (2005) 887-916; (b) R. O'Kennedy, R.D. Thornes, Coumarins: Biology, Applications, and Mode of Action, Wiley, Chichester, NY, 1997; (c) M.E. Riveiro, N. de Kimpe, A. Moglioni, et al., Coumarins: old compounds with novel promising therapeutic perspectives, Curr. Med. Chem. 17 (2010) 1325-1338. |
| [7] | S. Vilar, E. Quezada, L. Santana, et al., Design, synthesis, and vasorelaxant and platelet antiaggregatory activities of coumarin-resveratrol hybrids, Bioorg. Med. Chem. Lett. 16 (2006) 257-261. |
| [8] | O. Thastrup, J.B. Knudsen, J. Lemmich, K. Winther, Inhibition of human-platelet aggregation by dihydropyrano and dihydrofuranocoumarins, a new class of camp-phosphodiesterase inhibitors, Biochem. Pharmacol. 34 (1985) 2137-2140. |
| [9] | D.L. Yu, M. Suzuki, L. Xie, S.L. Morris-Natschke, K.H. Lee, Recent progress in the development of coumarin derivatives as potent anti-HIV agents, Med. Res. Rev. 23 (2003) 322-345. |
| [10] | (a) E. Budzisz, E. Brzezinska, U. Krajewska, M. Rozalski, Cytotoxic effects, alkylating properties and molecular modelling of coumarin derivatives and their phosphonic analogues, Eur. J. Med. Chem. 38 (2003) 597-603; (b) D. Cooke, R. O'Kennedy, Comparison of the tetrazolium salt assay for succinate dehydrogenase with the cytosensor microphysiometer in the assessment of compound toxicities, Anal. Biochem. 274 (1999) 188-194; (c) D. Egan, P. James, D. Cooke, R. OKennedy, Studies on the cytostatic and cytotoxic effects and mode of action of 8-nitro-7-hydroxycoumarin, Cancer Lett. 118 (1997) 201-211; (d) P. Hilgard, R.D. Thornes, Perspectives in cancer research anticoagulants in treatment of cancer, Eur. J. Cancer 12 (1976) 755-762; (e) U.S. Weber, B. Steffen, C.P. Siegers, Antitumor-activities of coumarin, 7-hydroxy-coumarin and its glucuronide in several human tumor cell lines, Res. Commun. Mol. Path. 99 (1998) 193-206. |
| [11] | C.A. Kontogiorgis, K. Savvoglou, D.J. Hadjipavlou-Litina, Antiinflammatory and antioxidant evaluation of novel coumarin derivatives, J. Enzym. Inhib. Med. Chem. 21 (2006) 21-29. |
| [12] | (a) H.S. Jung, P.S. Kwon, J.W. Lee, et al., Coumarin-derived Cu2+-selective fluorescence sensor: synthesis, mechanisms, and applications in living cells, J. Am. Chem. Soc. 131 (2009) 2008-2012; (b) X.G. Liu, J.M. Cole, P.G. Waddell, et al., Molecular origins of optoelectronic properties in coumarin dyes: toward designer solar cell and laser applications, J. Phys. Chem. A 116 (2012) 727-737. |
| [13] | H. Kaji, M. Yamada, K. Nozawa, K. Kawai, S. Nakajima, Synthesis of antifungal isocoumarins, Org. Prep. Proced. Int. 18 (1986) 253-262. |
| [14] | (a) S.J. Cai, F. Wang, C.J. Xi, Assembly of 3-substituted isocoumarins via a Culcatalyzed domino coupling/addition/deacylation process, J. Org. Chem. 77 (2012) 2331-2336; (b) T.L. Yao, R.C. Larock, Synthesis of isocoumarins and alpha-pyrones via electrophilic cyclization, J. Org. Chem. 68 (2003) 5936-5942. |
| [15] | (a) K. Katsumoto, C. Kitamura, T. Kawase, An indenone synthesis involving a new aminotransfer reaction and its application to dibenzopentalene synthesis, Eur. J. Org. Chem. (2011) 4885-4891; (b) R. Leardini, D. Nanni, A. Tundo, G. Zanardi, Novel [3+2] radical annulations of cyano-substituted aryl radicals with alkynes, Tetrahedron Lett. 39 (1998) 2441-2442. |