b Department of Chemical Engineering, Tsinghua University, Beijing 100084, China
Thermoexpandable microspheres consist of a drop of liquid hydrocarbon,encapsulated by a gas-proof polymeric shell. Before expansion,the typical diameter is about 20mm and the density is 1000 kg/m3. When the microspheres are heated to about 80- 110°C,the diameter increases to about 80mm and the density decreases to 20-30 kg/m3[1]. Thermoexpandable microspheres have proven to be a very useful product in a wide variety of applications,including as blowing agents or as light weight fillers, and in printing inks to capture the three-dimensional patterns on paper,wallpaper,and textiles,etc.[2, 3, 4].
The preparation method of thermoexpandable microspheres was first described in a patent of DowChemical Co. [5],and after that many patents and papers have published [6, 7, 8, 9, 10]. The microspheres are prepared by a suspension polymerization of droplets of a mixture of monomer and blowing agent. Acrylonitrile (AN) and methacrylonitrile (MAN) are generally used as polymerizable monomers [7, 10, 11]. There are few domestic reports on the subject and none used methyl methacrylate (MMA) as one of the monomers.
In this study,thermoexpandable polymeric microspheres were prepared by suspension polymerization with AN and MMA as monomers,i-butane as a blowing agent and ethoxylated trimethylolpropane triacrylate (EOTMTA) as a cross-linking agent. The properties of the thermoexpandable polymeric microspheres were studied. 2. Experimental
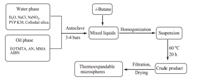
The thermoexpandable polymeric microspheres were prepared according to Scheme 1,and the detailed steps are described as follows:
|
Download:
|
| Scheme 1. Preparation process of thermoexpandable microspheres. | |
Water phase: sodium chloride (4.44 g),sodium nitrite (3.75 g, 2.5 wt% water solution),colloidal silica (18.0 g,30% solids, approx. 10 nm diameter,pH9.8) and PVP K30 (3.5 g,aqueous solution,20 wt%,MW= 30,000 g/mol) were dissolved in deionized water (220.8 g). After the aqueous phase was mixed,the pH value was adjusted to about 3.5 by adding hydrochloric acid (2.0 mol/L),and then the water phase was cooled to about 5°C. Oil phase: EOTMTA (0.67 g) and AIBN (0.70 g) were added to the monomers (AN: 47 g; MMA: 47 g; distilled before use) and stirred to dissolve,then the oil phase was cooled to about 5°C. Homogenization and polymerization process: the aqueous phase and oil phase were poured to an autoclave (1.0 L) and the autoclave was sealed. The system was purged with N2 for about 3 min to exclude the oxygen,then the autoclave was resealed and pressurized with N2 to about 3-4 bars. The pressure addition burette containingi-butane (38 g,28.5 wt% of oil phase) was connected with the autoclave,andi-butane was dropped to the autoclave under pressure provided by a nitrogen tank. Once i-butane was completely added,the mixture was sheared at 1200-1300 rpm for about 12 min. Then,the stirring speed was reduced to 230-250 rpm and the autoclave was heated to 60°C with a water bath to polymerize.
Work-up: after polymerization for 20 h,the autoclave was cooled to room temperature by ice water,and the pressure decreased slowly to normal pressure. Agglomerates and larger particles were removed from the dispersions using a 100mm sieve. The products were obtained using a vacuum filtration assembly with qualitative filter paper,and dried for more than 48 h at ambient temperature and pressure after thorough washing with water. 3. Results and discussion
3.1. Micromorphology
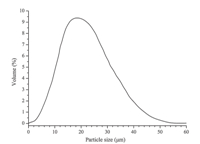
The micromorphology of microspheres was observed with polarized microscopy before and after expansion. The optical micrographs are shown in Fig. 1. The result of dynamic light scattering (Fig. 2) showed that the average diameter of the unexpanded microspheres (Fig. 1(a)) was 19.5mm,and it could be observed clearly that there was a small liquid oscillating drop centred in every microsphere. Apparently,the small liquid drop observed was the blowing agent (i-butane). The diameter of the expanded microspheres was about 40-80mm (Fig. 1(b)). The uneven size could be due to the different content of blowing agent in the microsphere and the variability of the current preparation method.

|
Download:
|
| Fig. 1. The polarized microscopy of the (a) unexpand microspheres and (b) expanded microspheres (120-130°C,2 min). | |
|
Download:
|
| Fig. 2. Particle size distribution of microspheres. | |
The density of the dried microspheres was about 0.455 g/mL. When the microspheres were heated to 120-130°C and maintained for about 2 min,the maximum volume was higher than 22 times of its original volume (Fig. 3). The weight loss of the expanded microspheres was about 11 wt%,which was mainly due to the loss of the water in the particles and the residual monomer. The density of the expanded microspheres was about 0.0167 g/mL (16.7 kg/m3). When the microsphere was heated in an bake oven from 70°C to 150°C at a rate of 2°C/min,the To.e.(temperature onsets of expansion), Tm.e.(temperature at maximum expansion volume) and To.s.(temperature onsets of shrinkage) were observed as 80°C,120-130°C and 140-145°C,respectively.

|
Download:
|
| Fig. 3. The graduated test tube photo of the unexpanded (right,0.5 mL) and expanded (left,120-130°C) microspheres. | |
Fig. 4 was the TGA curve of the microspheres. The weight loss of the microspheres at×100°C was about 7% (Fig. 4,I),which was due to the loss of the water and residual monomer in the microspheres. The weight loss at×120°C was about 20% (Fig. 4,II),which should be due to the loss of the blowing agent encapsulated in the microspheres. When the temperature was higher than theTgof the microspheres,the blowing agent would leak out from the softening polymer shell. From the results of TGA,it could be determined that the blowing agent content in microspheres was about 20 wt%, which was lower than that in the raw material.

|
Download:
|
| Fig. 4. TGA curve of the microspheres. | |
Thermoexpandable polymeric microspheres were prepared by suspension polymerization with AN and MMA as monomers and i-butane as a blowing agent. The diameter of the expandable microspheres increased from about 20mm (unexpanded) to 40-80mm (expanded) upon heating. The maximum expansion volume was higher than 22 times of the original volume and the density of expanded microspheres was about 16.7 kg/m3. To.e., Tm.e. and To.s.were 80°C,120-130°C and 140-145°C,respectively and the blowing agent content in microspheres was about 20 wt%. Acknowledgment
This work was supported by Shandong Provincial Natural Science Foundation,China (No. ZR2013EMM004).
| [1] | Y. Kawaguchi, T. Oishi, Synthesis and properties of thermoplastic expandable microspheres: the relation between crosslinking density and expandable property, J. Appl. Polym. Sci. 93 (2004) 505-512. |
| [2] | D. Lester, R.R. Alexander, Expandable polymeric coating compositions, United States Patent, US 4094685, 1978. |
| [3] | L.O. Svedberg, G. Hovland, T. Holmund, Method and expansion device for preparing expanded thermoplastic microspheres, United States Patent, US 20040176487, 2004. |
| [4] | M.X. Liu, L.H. Gan, W. Xiong, et al., Partially graphitic micro-and mesoporous carbon microspheres for supercapacitors, Chin. Chem. Lett. 24 (2013) 1037-1040. |
| [5] | J. Morehouse, R.J. Tetreault, Expansible thermoplastic polymer particles containing volatile fluid foaming agent and method of foaming the same, United States Patent, US 3,615,972, 1971. |
| [6] | J.L. Garner, Polymerization of styrene acrylonitrile expandable microspheres, United States Patent, US 3945956, 1976. |
| [7] | M. Jonsson, O. Nordin, E. Malmström, et al., Suspension polymerization of thermally expandable core/shell particles, Polymer 47 (2006) 3315-3324. |
| [8] | X.H. Lv, L.P. Wang, G. Li, et al., Preparation and characterization of optically functional hollow sphere hybrid materials by surface-initiated RATRP and "click" chemistry, Chin. Chem. Lett. 24 (2013) 335-337. |
| [9] | Y. Nishiyamaa, N. Uto, C. Sato, H. Sakurai, Dismantlement behavior and strength of dismantlable adhesive including thermally expansive particles, Int. J. Adhes. Adhes. 23 (2003) 377-382. |
| [10] | K. Yasuhiro, I. Yosuke, O. Kenjiro, et al., Heat-resisting expandable microspheres utilizing interactions between methacrylic acid and metallic salts, Kobunshi Ronbunshu 62 (2005) 36-43. |
| [11] | Y. Kawaguchi, Y. Itamura, K. Onimura, T. Oishi, Effects of the chemical structure on the heat resistance of thermoplastic expandable microspheres, J. Appl. Polym. Sci. 96 (2005) 1306-1312. |




