2,4,6-Trinitrotoluene (TNT),a leading example of a nitroaromatic explosive,detrimentally affects the environment and human health,and threatens the safety of society. Traditional assays for TNT detection,such as gas chromatography and mass spectroscopy (MS),are accurate. However,these assays cannot be field-deployed and need bulky and expensive instruments,time-consuming sample preparation,and well-trained technicians [1, 2, 3]. For onthe-spot detection of TNT,dramatic improvements in detection methods are essential.
Recently,nanomaterials with various functionalities have been widely employed as reliable analytical platforms for TNT. For example,nanomaterial-based optical sensors for TNT detection exhibit improved sensitivity and selectivity [4, 5, 6, 7, 8, 9]. A cysteinemodified Au nanoparticle (Au NPs) can serve as a surface-enhanced Raman probe for TNT recognition based on the formation of a Meisenheimer complex between TNT and cysteine [9],and amine group-functionalized mesoporous silica nanocomposites containing fluorescent polymers have been used as sensors to detect TNT by emission quenching [10]. Also,nanostructures containing fluorescent Ag nanoclusters (NCs) have been employed to sense and image TNT [11],and various quantum dots (QDs) have been exploited to detect TNT [7, 12, 8]. However,most of these strategies involve time-consuming nanomaterial preparation and functionalization and require special instruments. For promoting safety and environmental conscientiousness,visualized colorimetric probes enabling on-the-spot detection of TNT are preferred.
Visual colorimetric methods are extremely attractive because the detection results can be easily read out by the naked eye. The surface plasmon absorption band of gold nanoparticles,which is located in the visible region with a very high extinction coefficient (2.7×108L mol-1 cm-1 at 520 nm for 13 nm Au NPs),provides promising visualized colorimetric platforms [13, 14, 15, 16]. All these colorimetric sensors are based on the color change of Au NPs caused by their controllable dispersion/aggregation states. Au NPbased colorimetric probes are often achieved by carefully tailoring the surface chemistry of the Au NPs for identification of specific analytes. Particularly,cysteamine-functionalized Au NPs has been developed into a colorimetric sensor for determination of TNT [17].
The electron donor-acceptor (D-A) interaction between the primary amine of cysteamine and TNT allows aggregation of the initially dispersed Au NPs,resulting in color change from red to blue. Also,ethylenediamine (EDA)-capped Au NPs can serve as similar probes for TNT [18]. These probes for TNT involve surfacemodification of the Au NPs and a time-consuming separation process.
Here,a new strategy based on supramolecular chemistry has been described for colorimetric determination of TNT using unmodified Au NPs. MA alone readily makes dispersed red Au NPs aggregate into gray/blue assemblies while the preformed MA- TNT supramolecular disenable aggregation of the Au NPs,which still remain red in color. These new observations provide a facile colorimetric TNT assay in aqueous solution. By naked eye inspection,identification of TNT is established. By spectrophotometer tools,quantification of TNT is accomplished. In contrast to previous sensing strategies with surface-modified Au NPs,here a new sensing strategy for TNT has been developed with the advantages of convenience,rapidity,and cost effectiveness. 2. Experimental details 2.1. Materials
2,4,6-Trinitrotoluene (TNT) was obtained from the local security department. Chloroauric acid tetrahydrate (HAuCl4·4H2O),trisodium citrate dehydrate,and melamine were purchased from Sinopharm Chemical Reagent Co.,Ltd. (Shanghai, China) and used as received. All other chemicals were of analytical grade and were used without further purification. 2.2. Instruments
UV-vis spectra were recorded with a PerkinElmer Lambda 35 spectrophotometer. Transmission electron microscopy (TEM) images were taken with a JEM 2100F field emission transmission electron microscope,and atomic force microscopy (AFM) images were performed with a Bruker Dimension Icon atomic force microscope. Hydrodynamic diameter of the MA-TNT supramolecule was analyzed by dynamic light scattering (DLS) (zetasizer, Malvern Instruments Ltd). Resonance scattering spectrum was measured with a Hitachi F4500 fluorescence spectrophotometer. 2.3. Preparation of Au NPs
Au NPs were synthesized by the conventional reduction of HAuCl4with trisodium citrate. Typically,25 mL of trisodium citrate (38.8 mmol L-1 ) was injected into a boiling solution containing HAuCl4(250 mL,1 mmol L-1 ),and the mixed solution was further refluxed for another 15 min into a wine-red sample. After equilibrium to room temperature under stirring,the sample was dialyzed (cut off 1 kDa). The size of the citrate-capped AuNPs as determined by TEM image was about 13 nm. The particle concentration of the AuNPs (ca. 15 nmol L-1 ) was determined according to Beer’s Law using an extinction coefficient of ca. 2.43×108L mol-1 cm-1 at 520 nm [19]. 2.4. TNT determination
Au NP solution was 3-fold diluted with deionized water. The TNT stock solution was prepared with alcohol as solvent. For detection of TNT,100mL of the premixed MA and TNT solutions were first prepared. These mixtures contained constant amount of 1.2 mmol L-1 MA and various TNT concentrations such as 0,80, 250,500,1000,1200,and 1500mmol L-1 . Then these mixtures were added into 100mL of the as-diluted Au NPs. The change in color of the sensing system was observed with the naked eye,and the absorption spectrum of the sensing system was recorded with a UV-vis spectrometer. 3. Results and discussion
3.1. Supramolecule of melamine and trinitrotoluene
The electron donor-acceptor (D-A) interaction between different molecules enables the formation of supramolecular structures [20, 21, 22]. For instance,TNT is prone to form supramolecules through electron donor-acceptor interactions [22]. We found here that a supramolecule consisting of MA and TNT can form in aqueous solution through the similar electron D-A interaction between electron-rich MA and electron-deficient TNT. The dried MA-TNT supramolecules can be characterized by AFM analyses (Fig. 1A). These dried supramolecule nanoparticles are mostly spherical with diameters in 4.5-12 nm. No such nanoparticles were observed with the dried TNT or MA alone under the same conditions,presumably due to these small single molecules being coated evenly on the mica sheet substrate such that they are too small to be observed. Also,such supramolecules can be further characterized by DLS analyses,and a significant DLS spectrum appeared with the MA-TNT solutions. The measured hydrodynamic diameter distribution of the formed MA-TNT supramolecule was in the range of 5.0-18 nm (Fig. 1B). However, no such effective scattering spectrum was observed in solutions containing only MA or TNT,confirming that the scattering spectrum is caused by the formation of MA-TNT supramolecules. A melamine molecule contains three primary amine groups and its specific structure facilitates the electron donor-acceptor interaction with TNT.

|
Download:
|
| Fig. 1. (A) AFM image and (B) hydrodynamic diameter distribution of MA-TNT supramolecule derived from DLS analyses. (C) The resonance scattering spectra ofmelamine (1.2 mmol L-1 ) in the presence of various concentrations of TNT (from bottom to top: the concentration of TNT is 0,0.08; 0.4,0.6,0.9,1.2,and 2.4 mmol L-1 ,respectively),and (D) the plot of the RS intensity at 473 nmversusthe concentration of TNT. | |
Moreover,the stoichiometry of the MA-TNT supramolecule can be determined by resonance scattering analyses. Fig. 1C shows that the RS intensity of the system increases with increasing amounts of MA-TNT,which indicates the increasing of the amount of the as-formed MA-TNT supramolecules. When the mole ratio of MA to TNT is up to 1:1,however,the RS intensity approaches an obvious plateau,as shown in Fig. 1D. This result implies the stoichiometry of the MA-TNT supramolecule isca.1:1. The pH changes (from 5.0 to 10) of the system do not influence the RS intensity,and suggest that these supramolecules can form in a wide pH range. 3.2. Sensing mechanism
Citrate-stabilized Au NPs are wine red,whereas their aggregates appear gray,purple,or blue due to the shift of the surface plasmon absorption bands to a longer wavelength. Amines are well-known to bind well to noble metal particles [23]. MA molecules (contains triamine and triazine groups) can bind with Au NPs by formation of Au-N bonds and cross-link with Au NPs by offering multiple ligand sites. As depicted in Fig. 2A,the MA molecule can cross-link citrate-stabilized Au NPs,resulting in aggregation of Au NPs along with color change from red to gray/ blue [24, 25, 26]. The absorption spectrum of MA-induced Au NP aggregates is shown in Fig. 2B (solid curve). Compared with the initial Au NPs,the absorbance at 520 nm of the Au NP aggregates essentially decreased along with the appearance of a new absorbing peak at ca. 700 nm. The color of the MA-induced Au NP aggregates is gray/blue [Fig. 3A(a)] while that of the citrate-stabilized Au NPs is red. In contrast,the preformed MA-TNT supramolecules disenable aggregation of the Au NPs,and there is no obvious change in either the color or absorption spectrum of the Au NPs upon addition of the MA-TNT [Fig. 2B (dotted line) and Fig. 3A(e)].

|
Download:
|
| Fig. 2. (A) Schematic representation of the sensing mechanism of the proposed Au NPs probes for colorimetric detection of TNT. (B) The UV-vis absorption spectra of the Au NPs (3-fold dilution) in the absence (long dash line) and in the presence of MA-TNT supramolecules (formed by mixing MA and TNT both with 1.2 mmol L-1 ,dotted line) and only MA (solid line). MA enables aggregation of the Au NPs and changes its absorption spectrum while MA-TNT disenables aggregation of the Au NPs. The TEM images of the Au NPs in the presence of MA (C) and MA-TNT (D). | |
|
Download:
|
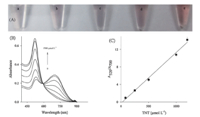
| Fig. 3. (A) The photographs of Au NPs upon the addition of MA (1.2 mmol L-1 ) mixed with various amounts of TNT (from a to e,the TNT concentration is 0,80,250,500,and 1000mmol L-1 ,respectively). (B) The TNT-dependent absorption spectra of Au NPs. From bottom to top,the TNT concentration is 0,80,250,500,1000,1200,and 1500mmol L-1 ,respectively. (C) Plot of the linear response of Au NPs toward TNT. Error bars were derived from triplicate separate measurements. | |
The aggregation and dispersion of the Au NPs by MA and MA- TNT were further studied by TEM analyses. As shown in Fig. 2C and D,Melamine allows aggregation of the Au NPs,while MA-TNT supramolecules disenable aggregation of the Au NPs. There is a significant color change with the alteration between dispersion and aggregation states of the Au NPs due to the sensitive interparticle-distance-dependent SPR properties of the Au NPs. Such color change allows Au NPs as colorimetric sensing platforms for various targets [27, 28, 29, 30, 31, 32]. 3.3. TNT determination
Fig. 3A shows that Au NPs exhibit different colors upon the addition of the solutions containing constant MA (1.2 mmol L-1 ) and various concentrations of TNT (0-1.0 mmol L-1 ). Due to the formation of MA-TNT supramolecules,the concentrations of the free MA subsequently reduce with the increasing of the TNT.
Melamine alone makes initially individual reddish Au NPs aggregate into gray/blue Au NP assemblies while the preformed supramolecule (MA-TNT) disenables aggregation of the Au NPs, and the Au NP solution still remains red. Therefore the colors of the Au NPs gradually change from gray to wine red with the increasing of TNT. These results indicate that the identification of TNT can be performed by naked eye inspection. Compared with the previous aggregation-based method (red-to-blue),the color change from gray/blue to red seems more eye-sensitive,especial in low concentration of targets [14]. Identification of TNT by naked eyes is promising for the on-the-spot detection.
By using photospectrometers as analytical tools,sensitive detection of TNT has been established. As indicated in Fig. 3B,the absorbance of the Au NPs at 520 nm significantly increased with the increasing of the concentration of TNT,while that at 700 nm decreased. To quantify TNT,absorbance ratio at 520 and 700 nm (A520/A700) of the sensing system is calculated. The plot of A520/A700versusTNT concentration is shown in Fig. 3C. A linear range of 80mmol L-1 to 1.2 mmol L-1 and a detection limit of 27mmol L-1 for TNT detection are obtained.
The selectivity of the proposed sensor was further tested. Common possible interferants,such as 2,4-dinitrotoluene,nitrobenzene,and toluene did not result in an obvious change in the color or in the spectrum of the MA-induced Au NP aggregates, suggested the proposed probes are applicable in practical applications. Further,experiments to test feasibility of the proposed modification-free Au NP probes for detection of TNT in real samples were also done. By spiking 250mmol L-1 TNT into the three pond water samples,an average recovery of 97% was obtained,implying that this probe is applicable. 4. Conclusions
In conclusion,a melamine-TNT supramolecule can form through their electron donor-acceptor interactions. Melamine alone readily makes initially dispersed red Au nanoparticles aggregate into gray/blue aggregates while the preformed supramolecule disenables aggregation of the Au NPs,thus providing a visual colorimetric assay for TNT. By naked eyes,identification of TNT is accomplished,and by using spectrophotometer tools, accurate TNT detection is accomplished. In contrast to the prior sensors based on the TNT-induced aggregation of the surface functionalized Au NPs,the proposed method based on melamine competitive binding between TNT and Au NPs simplifies and shortens the experimental process. Acknowledgment
s This work is supported by National Natural Science Foundation of China (No. 21375036),and the Open Project Program of Key Laboratory of Theoretical Chemistry and Molecular Simulation of Education,Hunan University of Science and Technology (No. E21201).
| [1] | D.G. Gehring, J.E. Shirk, Separation and determination of trinitrotoluene isomers by gas chromatography, Anal. Chem. 39 (1967) 1315-1318. |
| [2] | P. Sulzer, F. Petersson, B. Agarwal, et al., Proton transfer reaction mass spectrometry and the unambiguous real-time detection of 2,4,6 trinitrotoluene, Anal. Chem. 84 (2012) 4161-4166. |
| [3] | J. Yinon, H.G. Boettger, W.P. Weber, Negative ion mass spectrometry. New analytical method for detection of trinitrotoluene, Anal. Chem. 44 (1972) 2235-2237. |
| [4] | Y. Xia, L. Song, C. Zhu, Turn-on and near-infrared fluorescent sensing for 2,4,6-trinitrotoluene based on hybrid (gold nanorod)-(quantum dots) assembly, Anal. Chem. 83 (2011) 1401-1407. |
| [5] | H. Zhou, Z. Zhang, C. Jiang, et al., Trinitrotoluene explosive lights up ultrahigh raman scattering of nonresonant molecule on a top-closed silver nanotube array, Anal. Chem. 83 (2011) 6913-6917. |
| [6] | W.S. Zou, D. Sheng, X. Ge, J.Q. Qiao, H.Z. Lian, Room-temperature phosphorescence chemosensor and rayleigh scattering chemodosimeter dual-recognition probe for 2,4,6-trinitrotoluene based on manganese-doped ZnS quantum dots, Anal. Chem. 83 (2011) 30-37. |
| [7] | Y.Q. Wang, W.S. Zou, 3-Aminopropyltriethoxysilane-functionalized manganese doped ZnS quantum dots for room-temperature phosphorescence sensing ultratrace 2,4,6-trinitrotoluene in aqueous solution, Talanta 85 (2011) 469-475. |
| [8] | L. Fan, Y. Hu, X. Wang, et al., Fluorescence resonance energy transfer quenching at the surface of graphene quantum dots for ultrasensitive detection of TNT, Talanta 101 (2012) 192-197. |
| [9] | S.S.R. Dasary, A.K. Singh, D. Senapati, H. Yu, P.C. Ray, Gold nanoparticle based label-free SERS probe for ultrasensitive and selective detection of trinitrotoluene, J. Am. Chem. Soc. 131 (2009) 13806-13812. |
| [10] | L. Feng, H. Li, Y. Qu, C. Lu, Detection of TNT based on conjugated polymer encapsulated in mesoporous silica nanoparticles through FRET, Chem. Commun. 48 (2012) 4633-4635. |
| [11] | A. Mathew, P.R. Sajanlal, T. Pradeep, Selective visual detection of TNT at the subzeptomole level, Angew. Chem. Int. Ed. 51 (2012) 9596-9600. |
| [12] | E.R. Goldman, I.L. Medintz, J.L. Whitley, et al., A hybrid quantum dot-antibody fragment fluorescence resonance energy transfer-based TNT sensor, J. Am. Chem. Soc. 127 (2005) 6744-6751. |
| [13] | J.J. Feng, H. Guo, Y.F. Li, et al., Single molecular functionalized gold nanoparticles for hydrogen-bonding recognition and colorimetric detection of dopamine with high sensitivity and selectivity, ACS Appl. Mater. Interfaces 5 (2013) 1226-1231. |
| [14] | T. Lou, Z. Chen, Y. Wang, L. Chen, Blue-to-red colorimetric sensing strategy for Hg2+ and Ag+1 via redox-regulated surface chemistry of gold nanoparticles, ACS Appl. Mater. Interfaces 3 (2011) 1568-1573. |
| [15] | J.H. Lin, C.W. Chang, Z.H. Wu, W.L. Tseng, Colorimetric assay for S-adenosylhomocysteine hydrolase activity and inhibition using fluorosurfactant-capped gold nanoparticles, Anal. Chem. 82 (2010) 8775-8779. |
| [16] | Z. Zeng, S. Mizukami, K. Kikuchi, Simple and real-time colorimetric assay for glycosidases activity using functionalized gold nanoparticles and its application for inhibitor screening, Anal. Chem. 84 (2012) 9089-9095. |
| [17] | Y. Jiang, H. Zhao, N. Zhu, et al., A simple assay for direct colorimetric visualization of trinitrotoluene at picomolar levels using gold nanoparticles, Angew. Chem. Int. Ed. 47 (2008) 8601-8604. |
| [18] | D. Lin, H. Liu, K. Qian, et al., Ultrasensitive optical detection of trinitrotoluene by ethylenediamine-capped gold nanoparticles, Anal. Chim. Acta 744 (2012) 92-98. |
| [19] | S. Link, M.A. El-Sayed, Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods, J. Phys. Chem. B 103 (1999) 8410-8426. |
| [20] | B. Mukherjee, A.J. Pal, Write-once-read-many-times (WORM) memory applications in a monolayer of donor/acceptor supramolecule, Chem. Mater. 19 (2007) 1382-1387. |
| [21] | J. Rosenthal, J.M. Hodgkiss, E.R. Young, D.G. Nocera, Spectroscopic determination of proton position in the proton-coupled electron transfer pathways of donor-acceptor supramolecule assemblies, J. Am. Chem. Soc. 128 (2006) 10474-10483. |
| [22] | K.B. Landenberger, A.J. Matzger, Cocrystal engineering of a prototype energetic material: supramolecular chemistry of 2,4,6-trinitrotoluene, Cryst. Growth Des. 10 (2010) 5341-5347. |
| [23] | P.V. Kamat, Photophysical, photochemical and photocatalytic aspects of metal nanoparticles, J. Phys. Chem. B 106 (2002) 7729-7744. |
| [24] | W. Chen, H.H. Deng, L. Hong, et al., Bare gold nanoparticles as facile and sensitive colorimetric probe for melamine detection, Analyst 137 (2012) 5382-5386. |
| [25] | H. Chi, B. Liu, G. Guan, Z. Zhang, M.Y. Han, A simple, reliable and sensitive colorimetric visualization of melamine in milk by unmodified gold nanoparticles, Analyst 135 (2010) 1070-1075. |
| [26] | L. Li, B. Li, D. Cheng, L. Mao, Visual detection of melamine in raw milk using gold nanoparticles as colorimetric probe, Food Chem. 122 (2010) 895-900. |
| [27] | X. Su, R. Kanjanawarut, Control of metal nanoparticles aggregation and dispersion by PNA and PNA-DNA complexes, and its application for colorimetric DNA detection, ACS Nano 3 (2009) 2751-2759. |
| [28] | S. Hong, I. Choi, S. Lee, et al., Sensitive and colorimetric detection of the structural evolution of superoxide dismutase with gold nanoparticles, Anal. Chem. 81 (2009) 1378-1382. |
| [29] | R. Kanjanawarut, X. Su, Colorimetric detection of DNA using unmodified metallic nanoparticles and peptide nucleic acid probes, Anal. Chem. 81 (2009) 6122-6129. |
| [30] | S.H. Wu, Y.S. Wu, C.H. Chen, Colorimetric sensitivity of gold nanoparticles: minimizing interparticular repulsion as a general approach, Anal. Chem. 80 (2008) 6560-6566. |
| [31] | Z. Wang, R. Lévy, D.G. Fernig, M. Brust, Kinase-catalyzed modification of gold nanoparticles: a new approach to colorimetric kinase activity screening, J. Am. Chem. Soc. 128 (2006) 2214-2215. |
| [32] | M. Wang, X. Gu, G. Zhang, D. Zhang, D. Zhu, Continuous colorimetric assay for acetylcholinesterase and inhibitor screening with gold nanoparticles, Langmuir 25 (2009) 2504-2507. |




