b Zhongshan Torch Polytechnic, Zhongshan 528436, China;
c Shenzhen Engineering Laboratory of Lingnan Herbal Resource Development and Application, Shenzhen Institute for Drug Control, Shenzhen 518057, China
Stemonae Radix (‘Baibu’ in Chinese),the dried root ofStemona tuberosaLour. and relatedStemonaspecies (Stemonaceae family), has been used as a traditional Chinese medicine for antitussive and insecticide remedies for thousands of years [1, 2]. Plants of genus Stemonawere known to produce a variety of alkaloids with unique structures characterized by the presence of a pyrrolo[1, 2, a]azepine nucleus,which were believed to be responsible for the antitussive activity of the herb [3, 4, 5]. So far,more than 140 alkaloids had been isolated from genus Stemona[6].
During the course of studying the antitussive Stemona alkaloids in Stemonae Radix,we found that the chemical diversity in genus Stemonawas very significant [7, 8, 9]. Though alkaloids with pyrrolo[1, 2, a]azepine nucleus could be observed in all Stemonaspecies,the structural-types varied in different species. Furthermore,in the sameS. tuberosaspecies,four chemical-types were observed in different places of production [9].
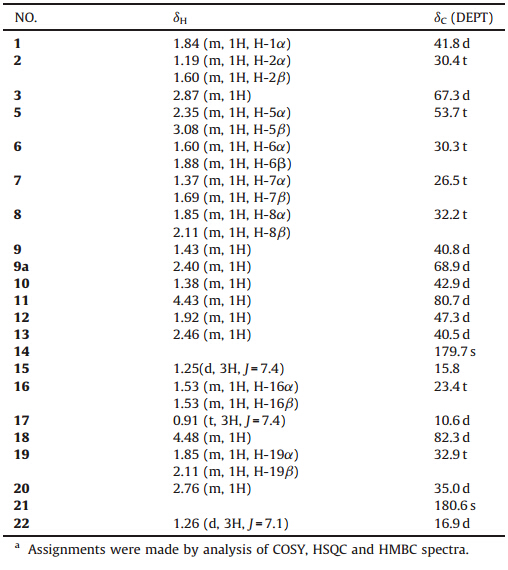
To further explore the chemical diversity ofS. tuberosa,we performed a phytochemical investigation on the ethanol extract of S. tuberosacollected in Yulin (Guangxi Province),leading to the isolation of one new alkaloid,tuberostemonine D (1) as well as seven known compounds (2-8),i.e. tuberostemonine (2) [9], tuberostemonine K (3) [9],tuberostemonine H (4) [10],didehydrotuberostemonin (5) [11],tuberostemoline (6) [12],bisdehydrostemonine (7) [13],and stemoninine (8) [14]. Compound 1 was a new stereoisomer of tuberostemonine. Herein,we reported the isolation and structure determination of 1. 2. Experimental
The dried roots ofStemona tuberosaLour. (3 kg) were powdered and extracted with 95% (v/v) EtOH at 90°C. The crude extract was acidified with dilute HCl (4%) to pH 1-2 and then partitioned between Et2O and H2O. The aqueous layer was separated and basified with CH3·H2O to pH 9-10 and extracted with Et2O to afford the crude alkaloids (26.8 g). The crude alkaloids (26.8 g) was subjected to silica gel column chromatography eluted with CHCL3-CH3OH gradient system (100:1-1:1) to afford 10 fractions (A-J). Fr. E (3.2 g) was separated by silica gel column using CHCL3-(CH3)2CO (30:1-0:30) as eluent to yield seven subfractions (Fr. E1-E7). Fr. E3 (183 mg) was subjected to preparative HPLC eluted with CH3CN-H2O (1:1) to give compounds 1 (34 mg) and 6 (13 mg). Fr. E6 (112 mg) was separated by Sephadex LH-20 column and preparative HPLC eluted by CH3CN- H2O (45: 65) to yield compounds 3 (24 mg),4 (23 mg) and 7 (18 mg). Fraction H (2.5 g) was separated by silica gel column using CHCL3-methanol (30:1-0:30) as the eluent to yield five subfractions (Fr. H1-H5). Fractions H4 (83 mg) was purified by preparative HPLC eluted by CH3CN-H2O (38: 62) to afford2 (22 mg) and 8 (14 mg). Similarly,fraction H5 (123 mg) was separated using the same method as H4 to afford5(24 mg).
|
Download:
|
| Fig. 1. The chemical structures of compounds 1-8. | |
Tuberostemonine D (1): colorless prism-like crystals; [α]D 26-11:8(c= 0.5,CH3OH); UV (CH3OH)lmax(loge) 208 (3.55) nm; IR (KBr,cm-1 )νmax 1761,1456,1199,1007; 1H NMR and 13C NMR data,see Table 1; HRESIMSm/z 376.2486 [M+H]+(calcd. for C22H33NO4,376.2482).
| Table 1 1H and 13C NMR spectral data of compound1(in pyridine-d5, 400 MHz for 1H).a |
X-Ray crystallographic analysis of 1.C22H33NO4,colorless blocks,Mr = 376.25,monoclinic,P21,a= 10.5516(4), b= 9.4061(3),c= 11.3265(4) Å ,β= 114.205(5),V= 1025.32(6) Å3, Z=2,dx= 1.216 mg/cm3,F(0 0 0) = 408,m(Cu-Ka) = 0.661 mm-1. Data collection was performed on a Gemini S Ultra using graphitemonochromated radiation (l= 1.54184 Å ); 2294 unique reflections were collected to umax= 62.71°,in which 2207 reflections were observed [F2>4s(F2)]. The crystal structure was resolved by direct methods using SHELXS-97 and refined by full-matrix leastsquares method on F2 using SHELXS-97. Non-hydrogen atoms were refined with anisotropic temperature factors. Hydrogen atoms bonded to carbons were placed at their geometrically ideal positions. FinalR= 0.0392,Rw= 0.1054 and S= 1.061. Crystallographic data for structure1have been deposited at the Cambridge Crystallographic Data Center (CCDC 980755). Copies of the data can be obtained free of charge on application to CCDC,12 Union Road,Cambridge CB2 1EZ,UK (Fax: +44 1223 336033; Email: deposit@ccdc.cam.ac.uk). 3. Results and discussion
Compound1was isolated as colorless prism-like crystals. Its molecular formula was established as C22H33NO4 by the positive quasi-molecular ion peak atm/z376.2486 [M+H]+in its HR-ESIMS with seven degrees of unsaturation. The IR band at 1761 cm-1 and 13C NMR datadC179.7 and 180.6 suggested the presence of twoglactone rings. The 1H NMR spectrum showed the presence of characteristic signals for one primary methyl atδH0.90 (t,3H, J= 7.4 Hz,H-17),two secondary methyls at δH 1.26 (d,3H, J= 7.1 Hz,H-22) and 1.25 (d,3H,J= 7.4 Hz,H-15),one methylene and two methines bearing a nitrogen atom at δH3.08 (m,1H,H-5b), 2.53 (m,1H,H-5a),2.40 (m,1H,H-9a) and 2.87(m,1H,H-3),and two oxymethines at δH4.43 (m,1H,H-11) and 4.48 (m,1H,H-18). The 13C NMR and DEPT spectra of1displayed 22 carbon signals, including two carbonyl carbons,10 methine carbons,seven methylene carbons,and three methyl carbons. A comparison of the 1H NMR and 13C NMR data of 1 with those of tuberostemonine (2) implied they have the same planar structure [9],suggesting that compound1might be an isomer of 2.
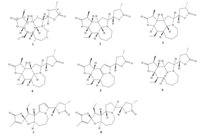
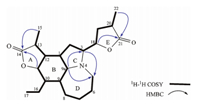
The full assignments and connectivities of all the H- and C-atom signals (Table 1) of 1 were further confirmed by a combination of 1H- 1H COSY,HSQC and HMBC spectral analyses. The 1H- 1HCOSY spectrum showed the spin systems involving H-9a↔H-9↔H-10↔H-11↔H-12↔H-13↔H-15; H-10↔H-16↔H-17 and H-18↔H-19↔H-20↔H-22,which were shown in bold face in Fig. 2.In the HMBC spectrum,the correlations between H-9a and C-3, between H-9a and C-5,as well as between H-3 and C-5 were observed,indicating C-3,C-9a and C-5 were connected via a nitrogen atom (N-4). The HMBC correlations between H-15 and C-14 and between H-11 and C-14 revealed the existence of ag-lactone ring (ring A). Similarly,the HMBC correlations between H-18 and C-11 and between H-22 and C-11 suggested the formation of anotherglactone ring (ring E),and the HMBC correlation between H-18 and C-3 indicated that this lactone ring was located at C-3. The relative configuration of1was determined by the ROESY spectrum,which showed NOE correlations H-9a↔H-3,H-9a↔H-9,H-9a↔H-12 and H-11↔H-12 (Fig. 3). These observations suggested H-3,H-9,H-9a,H-11 and H-12 were all b-oriented.
|
Download:
|
| Fig. 2. 1H- 1H COSY and key HMBC correlations of compound 1. | |
|
Download:
|
| Fig. 3. Key NOE correlations of compound 1. | |
Furthermore,NOE correlations between H-1↔H-10,and H-10↔H-13 indicated that those protons were alla-oriented.
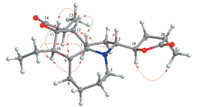
Moreover,NOE correlation between H-18 and H3-22 suggested they were on the same side of theg-lactone (ring E). However,the relative configuration of C-18 to C-3 still could not be determined as the free rotation of the single bond between C-3 and C-18. Fortunately,crystals of 1 suitable for single crystal X-ray diffraction analysis were obtained from the methanol solution. The relative configuration of1was unambiguously established by its X-ray crystal structure (Fig. 4). Besides,a final refinement of the CuKadiffraction data resulted in a small Flack parameter 0.2(3) permitting the assignment of the absolute configuration of1as 1R, 3R,9R,9aR,10R,11S,12S,13S,18R,and 20S.

|
Download:
|
| Fig. 4. X-ray crystal structure of1with atom-labeling scheme. | |
Up to now,there were eleven isomers of tuberostemonine reported (Supporting information). These isomers differ from each other by the configurations at C-1,C-3,C-9,C-9A,C-10,C-11,C-12, C-13,C-18 and C-20 [6, 15, 16]. Among these known isomers,3R configuration is rare except for tuberostemonine A. Compound1 represents the second isomer with the same 3Rconfiguration as tuberostemonine A,but the configuration at C-18 is S,which is different from tuberostemonine A. Thus compound1was a new stereoisomer of tuberostemonine,and was named as tuberostemonine D (Fig. 1).
Chemical diversity amongStemonagenus is very large [17]. Even in the same species ofS. tuberosa,chemical diversity is still significant. Previously,we studied the chemical diversity of S. tuberosa [9],and found that this herb from different places of production could be divided into four chemical types with tuberostemonine,neotuberostemonine,stemoninine and croomine as the major components,respectively. In the present study, compounds1-6belonged to the stenine-type (also belonged to the stichoneurine-type [15]) alkaloids and 7 and 8 belonged to the stemoninine-type alkaloids were identified. Thus first simultaneous isolation of stenine- and stemoninine types alkaloids added a new chemical type and increased the chemical diversity ofS. tuberosa. In addition,genusStemonais a rich source of stenine-type alkaloid [9],and genusStichoneuronis a rich source of stemoninine-type alkaloids [18]. Our previous study revealed the close taxonomic relationship between these two genera through parsimonious analysis of trnL DNA sequences [7]; however, chemotaxonomic evidence is weak. Our current results showed that stenine- and stemoninine types alkaloids could be simultaneously identified in Stemona plant,providing a significant chemotaxonomic clue confirming the close relationship between generaStemonaandStichoneuronas revealed by DNA analysis,and favored retaining them in the same Stemonaceae family. 4. Conclusion
From the roots of Stemona tuberosa,one new alkaloid tuberostemonine D (1) together with seven known compounds were isolated and identified by extensive spectroscopic methods. The molecular structure and absolute configuration of compound1 were further confirmed by single crystal X-ray diffraction analysis. First simultaneous isolation of stenine- and stemoninine types alkaloid not only added a new chemical type and increased the chemical diversity ofS. tuberosa,but also provided chemotaxonomic clues for the close relationship between generaStemonaand
Stichoneuronas revealed by DNA analysis,and favored retaining them in the same Stemonaceae family. Acknowledgments
This work was supported by the Fundamental Research Funds for the Central Universities (No. 11612603) and the Science and Technology Planning Project of Zhongshan City (No. 2013A3FC0325). Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.03.051.
| [1] | Pharmacopoeia Commission of People's Republic of China, The Pharmacopoeia of the People's Republic of China, Chemical Industry Publishing House, Beijing, 2005, p. 100. |
| [2] | Jiangsu New Medical College, Dictionary of Chinese Traditional Medicine, Shanghai People's Publishing House, Shanghai, 1977, pp. 858-861. |
| [3] | Y.T. Xu, P.C. Shaw, R.W. Jiang, et al., Antitussive and central respiratory depressant effects of Stemona tuberosa, J. Ethnopharmacol. 128 (2010) 679-684. |
| [4] | X. Zhou, P.H.H. Leung, N. Li, et al., Oral absorption and antitussive activity of tuberostemonine alkaloids from the roots of Stemona tuberosa, Planta Med. 75 (2009) 575-580. |
| [5] | X.Z. Yang, J.Y. Zhu, C.P. Tang, et al., Alkaloids from roots of Stemona sessilifolia and their antitussive activities, Planta Med. 75 (2009) 174-177. |
| [6] | R.A. Pilli, G.B. Rossoa, M.D.C.F.D. Oliveira, The chemistry of Stemona alkaloids: an update, Nat. Prod. Rep. 27 (2010) 1908-1937. |
| [7] | R.W. Jiang, P.M. Hon, Y.T. Xu, et al., Isolation and chemotaxonomic significance of tuberostemospironine-type alkaloids from Stemona tuberosa, Phytochemistry 67 (2006) 52-57. |
| [8] | R.W. Jiang, P.M. Hon, P.P.H. But, et al., Isolation and stereochemistry of two new alkaloids from Stemona tuberosa, Tetrahedron 58 (2002) 6705-6712. |
| [9] | R.W. Jiang, P.M. Hon, Y. Zhou, et al., Alkaloids and chemical diversity of Stemona tuberosa, J. Nat. Prod. 69 (2006) 749-754. |
| [10] | H.S. Chung, P.M. Hon, G. Lin, et al., Antitussive activity of Stemona alkaloids from Stemona tuberosa, Planta Med. 69 (2003) 914-920. |
| [11] | S.W. Liu, H.Z. Fu, W.H. Lin, Alkaloids from roots of Stemona tuberosa, Acta Pharm. Sin. 34 (1999) 372-375. |
| [12] | L.G. Lin, H.P.H. Leung, J.Y. Zhu, et al., Croomine-and tuberostemonine-type alkaloids from roots of Stemona tuberosa and their antitussive activity, Tetrahedron 64 (2008) 10155-10161. |
| [13] | L.G. Lin, Q.X. Zhong, T.Y. Cheng, et al., Stemoninines from the roots of Stemona tuberosa, J. Nat. Prod. 69 (2006) 1051-1054. |
| [14] | C. Kuo, T.T. Chu, A study of Stemona alkaloids: I, Acta Chim. Sin. 36 (1978), 291-196. |
| [15] | H. Greger, Structural relationships, distribution and biological activities of Stemona alkaloids, Planta Med. 72 (2006) 99-113. |
| [16] | J.P. Hua, D.H. Yang, W.H. Lin, et al., Alkaloids from the roots of Stemona tuberosa, Helv. Chim. Acta 92 (2009) 2125-2133. |
| [17] | K. Sumet, S. Johann, F. Susanne, et al., Structural relationships of Stemona alkaloids: assessment of species-specific accumulation trends for exploiting their biological activities, J. Nat. Prod. 74 (2011) 1931-1938. |
| [18] | R.A. Ramli, W. Lie, S.G. Pyne, Alkaloids from the roots and leaves of Stichoneuron halabalensis and their acetylcholinesterase inhibitory activities, Nat. Prod. Commun. 8 (2013) 695-698. |