b Department of Chemistry, Shahreza Branch, Islamic Azad University, Isfahan, Iran
Cysteamine is an aminothiol compound used as a drug for the treatment of cystinosis [1]. The deficiency of a cystine carrier in the lysosomal membrane leads to cystine accumulation within the lysosomes,ultimately crystallizing in vital organs such as the liver, kidney,spleen,intestines,and cornea. A number of long term clinical trials have shown that cysteamine administration (as cysteamine hydrochloride) stabilizes renal function,delays glomerular deterioration and improves linear growth [2]. The cysteamine and its disulfide,cystamine,have been shown to be neuroprotective in a number of cell culture and animal models [3]. So,determination of this drug is very important in pharmaceutical and biological compounds.
Carbon based nanomaterials,such as carbon nanotubes (CNTs) or graphene,are presently in the forefront of materials research in electrochemical and other fields [4, 5]. They are recognized as a new class of nano-materials that have had a profound impact on a wide range of applications. Research over the past decade has revealed that the CNTs constituted a new form of carbon materials that are finding striking applications in many fields,such as energy conversion and storage [6],chemical actuators [7],and chemical sensing [8, 9, 10].
In this study,the application of ISPT was initially described as a suitable mediator in the electrocatalysis and voltammetric determination of CA in an aqueous buffer solution. In sequence, in order to demonstrate the catalytic ability of the modified electrode in the electro-oxidation of CA in real samples,the method was employed for the voltammetric determination of CA in urine and drug samples. 2. Experimental
We used a Potentiostat/Galvanostat (m-Autolab (Eco Chemie, the Netherlands) coupled whit a Pentium IV personal computer connected to a HP laser jet 6L printer,and experiments were performed in a three compartment cell. A conventional threeelectrode cell assembly consisting of a platinum wire as an auxiliary electrode and an Ag/AgCl/KCl (sat.) electrode as a reference electrode was used. 3. Results and discussion
The working electrode was a modified multiwall carbon nanotube paste electrode. The electrochemical behavior of the ISPT was characterized by cyclic voltammetry. Fig. 1 (inset) shows the cyclic voltammograms of ISPT at MWCNTPE in the PBS (pH 4.0) at various scan rates. The experimental results showed well defined and reproducible anodic and cathodic peaks related to the ISPT(red)/ISPT(ox) redox couple with a quasireversible behavior and with a peak separation potential ofDEp(Epa×Epc) = 254 mV. These cyclic voltammograms were used to examine the variation of the peak currentsversusthe square root of potential scan rates. The plot of the anodic peak current was linearly dependent onn1/2with a correlation coefficient of 0.9906 at all scan rates (Fig. 1).

|
Download:
|
| Fig. 1. Plot of Ipa versus v1/2for the oxidation of 200mmol/L ISPT at a surface of MWCNTPE. Inset: cyclic voltammograms at various scan rates,1,5; 2,10; 3,15; 4, 20; 5,60; 6,100; 7,150 and 8,200 mV/s in 0.1 mol/L PBS (pH 4.0). | |
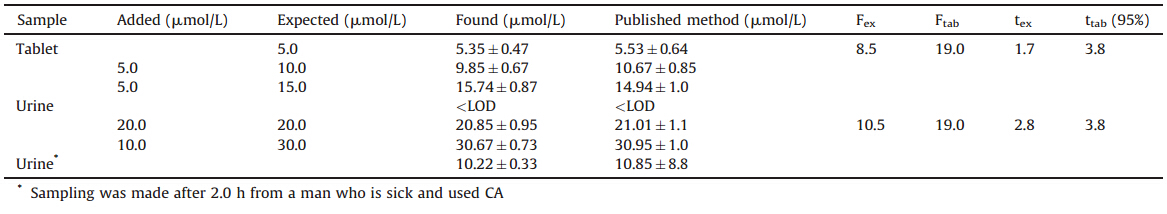
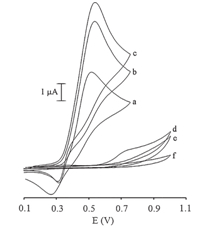
One objective of the present work was to develop a modified electrode capable of electro-catalytic oxidation of CA. The cyclic voltammetric responses for the electrochemical oxidation of 100mmol/L of CA at MWCNTPE and at carbon paste electrode (CPE) in the presence of 200mmol/L ISPT are shown in Fig. 2,curves c and b,respectively. Curve d and curve e (Fig. 2) are the same as curve c and curve b,but only without the mediator. Curve a (Fig. 2) shows the cyclic voltammogram of ISPT (200mmol/L) at a surface of MWCNTPE in the buffer solution (pH 4.0). As can be seen,the anodic peak potentials for the oxidation of CA in the presence of mediator at both MWCNTPE and CPE (curves c and b) are about 536 mV. On the other hand,the anodic peak potential for CA oxidation at the MWCNTPE,and unmodified CPE are 705 mV and 746 mV,respectively. Similarly,when we compared the oxidation of CA in the presence of mediator at the surface of the MWCNTPE (Fig. 2c) and at CPE (Fig. 2b),an enhancement of the anodic peak current was found to occur at the MWCNTPE. In other words,the data obtained clearly show that the combination of carbon nanotubes and mediator definitely improve the characteristics of the electrode for the oxidation of CA,by increasing the sensitivity and decreasing its overpotential,respectively. Based on these results,the following catalytic diagram (EC/catalytic mechanism) describes the voltammetric response of the electrochemical oxidation of CA in the presence of mediator at the surface of MWCNTPE (Scheme 1) [11, 12, 13].
|
Download:
|
| Fig. 2. Cyclic voltammograms of (a) 200mmol/L ISPT at the surface of MWCNTPE in 0.1 mol/L PBS (pH 4.0),(b) 200mmol/L ISPT + 100mmol/L CA at the surface of carbon paste electrode,(c) 200mmol/L ISPT + 100mmol/L CA at the surface of MWCNTPE,(d) 100mmol/L CA at the surface of MWCNTPE,(e) 100mmol/L CA at the surface of carbon paste electrode,(f) for the buffer solution at the surface of unmodified electrode (carbon paste electrode); scan rate of 20 mV/s. | |
|
Download:
|
| Scheme 1. The role of ISPT on the oxidation of CA. | |
In scan rate investigation,we observed a linear variation of the peak current for CA at the surface of the modified electrode in the presence of ISPT with the square root of scan rate (v1/2 )r2 = 0.9904. This result clearly indicates a diffusion-controlled electro-oxidative process [14, 15, 16, 17, 18]. To obtain information about the ratedetermining step,the Tafel plot was drawn,as derived from points in the Tafel region of the cyclic voltammogram. The slope of the Tafel plot was equal to 2.3RT/n(1-a)F,which came up to 6.77. Therefore,we obtained the mean value ofa,equal to 0.6.
Double potential step chronoamperometry was used with MWCNTPE in the presence of ISPT to determine the diffusion coefficient of CA. We have determined the diffusion coefficient,D, of CA using the Cottrell equation:

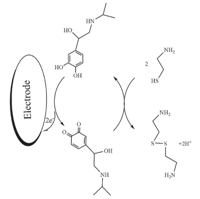
According to the above equation,we calculated a diffusion coefficient of 6.05×10-5 cm2 /s for CA. Linear sweep voltammetry (LSV) was used to determine CA (Fig. 3 Inset). The LS voltammograms clearly showed two linear dynamic ranges; the plot of the peak current versus CA concentration was linear for 0.3-14mmol/L with a regression equation of Ip (μA) = (0.0845±0.0023)CCA+ (4.8756±0.5150) (r2= 0.9921,n= 11) and for 14-450mmol/L of CA,the regression equation was Ip (μA) = (0.0047±0.0001)CCA+ (6.0825±0.8491) (r2= 0.9917,n= 11) (Fig. 3). The detection limit (LOD) was 0.09mmol/L CA according to the definition of LOD = 3sb/m(where sbis the standard deviation of the blank signal (n= 9) andmis the slope of the calibration).
|
Download:
|
| Fig. 3. The plots of the electrocatalytic peak current as a function of CA concentration. Inset shows the LSVs of modified electrode in the presence of mediator in 0.1 mol/L phosphate buffer solution (pH 4.0) containing different concentrations of CA. From inner to outer correspond to 0.3,3.0,5.0,6.0,7.0,8.0,9.0, 10.0,11.0,12.0,14.0,30.0,50,70,100,150,200,250,300,400 and 450.0mmol/L of CA. | |
The result shows that ascorbic acid could be the only interference or the determination of CA using this modified electrode. The interference by ascorbic acid can be minimized using ascorbic oxidase enzyme,which exhibits high selectivity to oxidation of ascorbic acid,if necessary.
Determination of CA in urine and tablet samples was examined for demonstrating the ability of the modified electrode to the determination of CA in real samples. Standard addition method was used for calculating the CA concentration. The proposed method was also compared with a published method [3] for checking the accuracy of the results. The results are given in Table 1 indicating that the modified electrode retained its efficiency for the determination of in real samples with satisfactory results. 4. Conclusion
In conclusion,this study provides for the construction of a chemically modified carbon paste electrode by incorporating multiwall carbon nanotubes as a suitable electrochemical sensor in the presence of ISPT as a mediator for CA determination at trace level. The results show that the oxidation of CA is catalyzed at pH 4.0,whereas the peak potential of CA is shifted by 210 mV to a less positive potential at the surface of the MWCNTPE in the presence of mediator. The proposed method is a selective,simple and precise method for voltammetric determination of CA in real samples,such as drug and urine,as low as 0.09mmol/L CA.
| Table 1 Determination of CA in pharmaceutical and urine samples (n = 3). |
The authors wish to thank Majlesi Branch,Islamic Azad University,Isfahan,Iran for their support.
| [1] | A. Taherkhani, H. Karimi-Maleh, A.A. Ensafi, et al., Simultaneous determination of cysteamine and folic acid in pharmaceutical and biological samples using modified multiwall carbon nanotube paste electrode, Chin. Chem. Lett. 23 (2012) 237-240. |
| [2] | M. Keyvanfard, S. Sami, H. Karimi-Maleh, K.H. Alizad, Electrocatalytic determination of cysteamine using multiwall carbon nanotube paste electrode in the presence of 3,4-dihydroxycinnamic acid as a homogeneous mediator, J. Braz. Chem. Soc. 24 (2013) 32-39. |
| [3] | A.A. Ensafi, H. Karimi-Maleh, A voltammetric sensor based on modified multiwall carbon nanotubes for cysteamine determination in the presence of tryptophan using p-aminophenol as a mediator, Electroanalysis 22 (2010) 2558-2568. |
| [4] | B.J. Sanghavi, S. Sitaula, M.H. Griep, et al., Real-time electrochemical monitoring of adenosine triphosphate in the picomolar to micromolar range using graphenemodified electrodes, Anal. Chem. 85 (2013) 8158-8165. |
| [5] | B.J. Sanghavi, P.K. Kalambate, S.P. Karna, A.K. Srivastava, A. Srivastava, Voltammetric determination of sumatriptan based on a graphene/gold nanoparticles/Nafion composite modified glassy carbon electrode, Talanta 120 (2014) 1-9. |
| [6] | G.X. Wang, J. Ahn, J. Yao, et al., Preparation and characterization of carbon nanotubes for energy storage, J. Power Source 119 (2003) 16-23. |
| [7] | M.A. Khalilzadeh, H. Karimi-Maleh, A. Amiri, F. Gholami, R. Motaghed Mazhabi, Determination of captopril in patient human urine using ferrocenemonocarboxylic acid modified carbon nanotubes paste electrode, Chin. Chem. Lett. 21 (2010) 1467-1470. |
| [8] | B.J. Sanghavi, A.K. Srivastava, Simultaneous voltammetric determination of acetaminophen, aspirin and caffeine using an in situ surfactant-modified multiwalled carbon nanotube paste electrode, Electrochim. Acta 55 (2010) 8638-8648. |
| [9] | B.J. Sanghavi, A.K. Srivastava, Adsorptive stripping differential pulse voltammetric determination of venlafaxine and desvenlafaxine employing Nafion-carbon nanotube composite glassy carbon electrode, Electrochim. Acta 56 (2011) 4188-4196. |
| [10] | M.L. Yola, N. Atar, A novel voltammetric sensor based on gold nanoparticles involved in p-aminothiophenol functionalized multi-walled carbon nanotubes: application to the simultaneous determination of quercetin and rutin, Electrochim. Acta 119 (2014) 24-31. |
| [11] | J.B. Raoof, R. Ojani, H. Karimi-Maleh, Electrocatalytic oxidation of thiosulfate at 2,7-bis(ferrocenylethyl)-fluoren-9-one-modified carbon paste electrode (2,7-BFEFMCPE): application to the catalytic determination of thiosulfate in real sample, Chin. Chem. Lett. 21 (2010) 1462-1466. |
| [12] | A.A. Ensafi, H. Karimi-Maleh, S. Mallakpour, M. Hatami, Simultaneous determination of N-acetylcysteine and acetaminophen by voltammetric method using N-(3,4-dihydroxyphenethyl)-3,5-dinitrobenzamide modified multiwall carbon nanotubes paste electrode, Sens. Actuators B 155 (2011) 464-472. |
| [13] | H. Karimi-Maleh, P. Biparva, H. Karimi-Maleh, A novel modified carbon paste electrode based on NiO/CNTs nanocomposite and (9,10-dihydro-9,10-ethanoanthracene-11,12-dicarboximido)-4-ethylbenzene-1,2-diol as a mediator for simultaneous determination of cysteamine, nicotin amide adenine dinucleotide and folic acid, Biosens. Bioelect. 48 (2013) 270-275. |
| [14] | A.L. Sanati, H. Karimi-Maleh, A. Badiei, P. Biparva, A.A. Ensafi, A voltammetric sensor based on NiO/CNTs ionic liquid carbon paste electrode for determination of morphine in the presence of diclofenac, Mater. Sci. Eng. C 35 (2014) 379-385. |
| [15] | M. Elyasi, M.A. Khalilzadeh, H. Karimi-Maleh, High sensitive voltammetric sensor based on Pt/CNTs nanocomposite modified ionic liquid carbon paste electrode for determination of Sudan I in food samples, Food Chem. 141 (2013) 4311-4317. |
| [16] | A.A. Ensafi, H. Karimi-Maleh, S. Mallakpour, et al., Highly sensitive voltammetric sensor based on catechol-derivative-multiwall carbon nanotubes for the catalytic determination of captopril in patient human urine samples, Colloid. Surf. B 87 (2011) 480-488. |
| [17] | T. Tavana, M.A. Khalilzadeh, H. Karimi-Maleh, et al., Sensitive voltammetric determination of epinephrine in the presence of acetaminophen at a novel ionic liquid modified carbon nanotubes paste electrode, J. Mol. Liquid 168 (2012) 69-74. |
| [18] | J.B. Raoof, R. Ojani, H. Karimi-Maleh, Carbon paste electrode incorporating 1-[4-(ferocenyl ethynyl)phenyl]-1-ethanone for electrocatalytic and voltammetric determination of tryptophan, Electroanalysis 20 (2008) 1259-1262. |