b State Key Laboratory of Chemical Resource Engineering, Beijing University of Chemical Technology, Beijing 100029, China
Over the past 50 years,polybrominated diphenyl ethers (PBDEs) were widely used as flame retardant in building materials, electronics,furnishings,foods,airplanes,plastics,polyurethane foams,and textiles [1, 2, 3, 4, 5]. The core structure of PBDEs was a diphenyl ether scaffold,which contains 10 hydrogen atoms. Every hydrogen atom can be replaced by a bromine atom,resulting in 209 congeners. PBDEs are stable,highly persistent,and easily bioaccumulated. Most PBDEs are difficult to degrade naturally [6, 7]. Moreover,they can migrate over a long distance,and integrate into food chains [5, 8]. Therefore,they have attracted increasing attention in the ecosystems because of the health hazards of these chemicals. PBDEs have been found in the environment,animal tissues and bloods,as well as in humans [9, 10]. Many studies have shown that PBDEs can reduce human fertility and display neurotoxicity [11]. Because of their toxicity and persistence,four kinds of PBDEs (TeBDE,PeBDE,HxBDE and HpBDE) were restricted under the Stockholm Convention,a treaty to control and phase out major persistent organic pollutants (POPs).
PBDEs are widely used in various products and can easily diffuse into the surrounding,especially in waters. For example, PBDEs from the fabrication and application of plastics,electronics, textiles,automotive,furniture,etc.,can all get into water [8, 12]. On the other hand,PBDEs can be resorbed by the environment through the discharge and disposal of sewage and sludge,becoming another source of PBDEs in the environment [13]. Large amount of persistent pollutants are poured into aquatic systems,which is the most important natural solvent. It is reported that PBDE concentrations in discharged effluent range from 4 pg/L to 20,000 pg/L [14]. Therefore,determination of PBDEs in water is of great importance and the results may be alarming. However, little is known about PBDEs in water because PBDEs are difficult to process by traditional technologies due to their congeners and degradation [15, 16]. Highly brominated diphenyl ethers such as NoBDE and DeBDE can easily lose one,two or even more bromine atoms to form lower brominated diphenyl ethers in the complex environment by biodegradation or photodegradation [17, 18, 19]. Moreover,little PBDEs would diffuse into water because they prefer sludge rather than sewage due to their high octanol-water partition coefficient [6, 13]. Rayne’s group has reported that the total concentration of lower brominated PBDEs (except BDE-209, as expressed as PBDEs) was 140 ng/L in the sewage plant influent [15]. However,it is only about 2.9-46 ng/L in the effluent. The influent concentration of BDE-209 was 169 ng/L. In contrast,the effluent concentration was 6 ng/L. Therefore,a large volume of sample was required.
Large volumes of sample can enhance matrix influence and suppress the signal. Thus,various sample pretreatment methods were developed. Soxhlet extraction,a traditional sample pretreatment for PBDEs,was usually laborious and time-consuming. Many researchers reported that PBDEs can be extracted by solid phase extraction/solid phase microextraction (SPE/SPME) [20, 21]. However, it has some drawbacks,such as high cost,sample carry-over, and time-declining performance. More importantly,SPE/SPME was not suitable for large volume extraction because the sample would be overloaded,and extra instrument such as a pump was needed. Dispersive liquid-liquid microextraction (DLLME) was also developed because it was simple and highly effective,but it was easy to over-extraction and some matrix could easily condensed [22, 23]. Therefore,liquid-liquid extraction (LLE) as a reliable and simple method was often used to pretreat water samples in large volumes [14, 24]. Moreover,due to the extremely low level of the targeted compounds and the large number of interfering substances, analytical methods for PBDEs determination are difficult to develop. To overcome these difficulties,highly selective detection methods and/or tedious pretreatment methods are demanded [25, 26]. GC was used to separate the targets. A mass spectrometer that enabled the identification of chemical structure [27, 28, 29] was used as a detector. Thus,an internal standard coupled with GC-MS is developed to provide a reliable quantification and to accurately determine the concentrations of these targets.
The purpose of this work is to establish an accurate and reliable method for the determination of PBDEs in water. Ten PBDEs with different degrees of bromination were analyzed by liquid-liquid extraction coupled with GC-MS (Fig. 1). The developed method was successfully applied to analyze PBDEs in river water. Moreover,the possible behavior of PBDEs in environment was proposed.

|
Download:
|
| Fig. 1.Procedure for the determination of PBDEs in river water based on LLE-SPE and GC-MS. LLE indicated liquid-liquid extraction. SPE depicted solid phase extraction by silica gel column. | |
Acetone and hexane (HPLC grade) were purchased from J. K. Baker Corporation. Water was obtained by purification of deionized water through a Milli-Q system (Millipore,Bedford,MA, USA). All PBDEs were purchased from Wako (Osaka,Japan,purity 98%). Mixtures of PBDEs except DeBDE were made in a concentration range of 0.1-500.0 ng/L. DeBDE was prepared in the concentration range of 1.0-5000.0 ng/L. 2.2. Sample collection and pretreatment
Water samples were collected in glass bottles at the position above 60 cm above the riverbed. The river is located at the east of YuanMingYuan. To validate the LLE method,a 100 mL water sample spiked with 2.00 ng/L PBDEs was used. The solution was filtered through a 0.45 μm membrane,and then extracted with 3 × 20 mL petroleum ether-hexane (1:2). The elute solution was dried over Na2SO4. Then the extraction solvent was condensed to 2 mL by nitrogen blowing.
The extraction solution was subjected to column chromatography by packing 6.0 g of silica gel particle in a column (15 cm × 10 mm I.D.). 0.5 cm3 of absorbent cotton was placed at the top and the bottom of the column. Before using,the silica gel was activated at 140 8C overnight. The column was equilibrated with 30 mL of petroleum ether-hexane (1:2) solvent. Then the sample was introduced. The column was eluted with 2 × 15 mL of the petroleum ether-hexane (1:2) solvent. All elution solvents were combined and dried over anhydrous Na2SO4. Then,the eluted solvent was condensed to 500 μL by the usage of 1 mL/min nitrogen gas. Finally,1 μL of the extraction was injected into the GC-MS. The pretreatment procedures were the same as those for the un-spiked samples. 2.3. GC-MS system for the determination of PBDEs
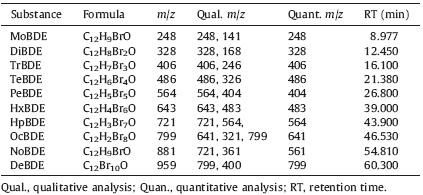
The ten PBDEs were determined using a Shimazu GC/MS QP2010 system. The GC system was equipped with an electronic pressure controller,a programmable-temperature vaporizer (PTV) and an autosampler. A 30 m × 0.25 mm I.D Rtx-ris capillary column with a film thickness of 0.25 μm was selected as the GC column. In these cases,the stationary phase of the analytical columns was 5% phenyl,95% dimethylpolysiloxane. The PVT started from 110 8C for the initial 1 min,was increased to 250 8C with a rate of 12 8C/min and maintained at 250 8C for 1 min. After that,the temperature was increased at a rate of 1.5 8C/min to 320 8C,and was kept at 320 8C for 5 min. The mass spectrometer was operated in electron ionization (EI) in the scan mode at them/z range from 100 to 1000 for all PBDEs. Dwell times were set at 50 ms. Helium was used as mobile phase. The temperatures of ion source,injection,and interface were set at 250,320 and 320 8C, respectively. The electron energy was 70 eV. The style of injection was splitless and the injected volume was 1 μL. A quantitative analysis of PBDEs was carried out by selecting a specific mass for each analyte,that is,the selected-ion monitoring (SIM) mode. Table 1 listed the retention times and m/z ratios of the quantification and confirmation ions of all PBDEs.
| Table 1 GC-MS parameters for PBDE determination. |
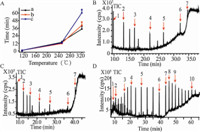
PBDEs with different chemical structures and polarity allowed us to separate them using column chromatography. Therefore,the mixture of ten congeners of PBDEs from mono- to decabromination were separated by GC-MS. Temperature of vaporizing was the key element for GC resolution. Three PVT programs were studied (Fig. 2A). Fig. 2B-D showed the total ion chromatograms (TIC) corresponding to the three PVTs of standard PBDEs at 2 ng/mL (expect DeBDE). The DeBDE was at 20 ng/L. The peaks of NoBDE and DeBDE disappeared in Fig. 2B,and DeBDE lost in Fig. 2C. But, Fig. 2D displayed the peaks of NoBDE and DeBDE. It was deduced that the accelerating programming rate of temperature will lead to target intensity loss. Therefore,PVT 3 was selected for the subsequent experiments because the ten PBDEs were all observed using this protocol. These results also indicated that 30 mlength of capillary column offered sufficient resolution for PBDEs determination. Moreover,the retention times (RTs) of ten PBDEs are listed in Table 1.
|
Download:
|
| Fig. 2.Total ion chromatograms (TIC) of PBDEs by GC-MS. (A) Three kinds of PTV for TIC. (B) TIC for a PTV. (C) TIC for b PTV. (D) TIC for c PTV. The concentration of PBDEs was 2 ng/L except DeBDE. The concentration of DeBDE was 20 ng/L. 1: MoBDE; 2: DiBDE; 3: TrBDE; 4: TeBDE; 5: PeBDE; 6: HxBDE; 7: HpBDE; 8: OcBDE; 9: NoBDE; 10: DeBDE. | |
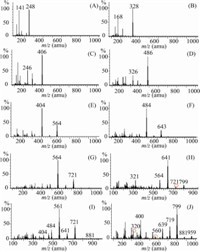
The qualitative and quantitative analysis of PBDEs was based on their characteristic fragmentation pattern in EI mass spectra. Fig. 3 showed that the SIM chromatograms of PBDEs from mono- to deca-bromination,and the productions of PBDEs were listed in Table 1. Fig. 3 showed that low brominated PBDE congeners were difficult to debrominate,and preserved their parent ions. However, the higher brominated PBDEs were difficult to retain their parent ions and easy to lose the bromine. Fig. 3H-J showed that OcBDE, NoBDE and DeBDE were deprived of bromine. The debromination of OcBDE led to HPBDE (m/z = 721,564) and PeBDE (m/z = 564, 404),and the degradation of NoBDE produced HpBDE (m/z = 721, 561),HpBED (m/z = 641,484),and/or even to PeBDE (m/z = 561, 404). The DeBDE lost bromine easily to generate OcBDE (m/z = 799, 639,320) and PeBDE (m/z = 560,400). Moreover,the intensity of fragment ion was much higher than that of its parent ion, suggesting that DeBDE,NoBDE,and OcBDE were sensitive to the temperature,and they had higher susceptibility for degradation in the GC system.
|
Download:
|
| Fig. 3.The selected ion monitoring chromatograms of PBDEs from MoBDE to DeBDE. | |
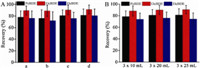
In order to investigate the feasibility of the method,100 mL of pure water spiked with 2.0 ng/mL PeBDE and OcBDE,and 20.0 ng/mL DeBDE was used to study the performance of our system. Conditions of the extraction solvent and its volume were optimized. Extraction solvent was a key factor contributed to the isolation and purification efficiency of the targeted analytes from river water. When choosing the solvent,some principles should be considered. For example,the solvent should have a high extraction efficiency for the analytes,and low dissolution in water. In this case,carbon tetrachloride (CCl4),hexane (C6H14) were selected. 100 mL of sample solution was extracted using 3 × 20 mL extraction solvents containing 1:1 or 1:2 petroleum ether to hexane,or petroleum ether to carbon tetrachloride. As shown in Fig. 4A,1:2 petroleum ether-hexane had the highest recovery than other extraction solutions. It was deduced that the polarity of 1:2 petroleum ether-hexane was more suitable for PBDEs than other solutions. Moreover,the polarity of 1:2 petroleum ether-hexane would also be more appropriate for OcBDE,leading to a better recovery. Another reason for low recovery of DeBDE may be due to the low level of degradation of DeBDE. Herein,1:2 petroleum ether-hexane was chosen for the next experiments.
In addition,the extraction volume was optimized. The relationship between the recovery of analytes and extraction volume was investigated in solutions with a volume of 3 × 10 mL, 3 × 20 mL,and 3 × 25 mL (Fig. 4B). The recovery increased proportionally to the extraction volume. However,when the extraction volume increased to 3 × 25 mL,the response of DeBDE decreased to some degree. However,the recovery of the other two PBDEs changed little. As shown in Fig. 4B,from 3 × 10 mL to 3 × 25 mL,response of none of the analytes in extraction solutions with large volume and low concentration had remarkable difference. Considering the enrichment time of PBDEs,3 × 20 mL petroleum ether-hexane (1:2) was selected for the next experiment.
|
Download:
|
| Fig. 4.Optimization of LLE by extraction solvent (A) and extraction volume (B). (a) 1:1 petroleum ether-CCl4; (b) 1:2 petroleum ether-CCl4; (c) 1:1 petroleum ether- C6H14; (d) 1:1 petroleum ether-C6H14. | |
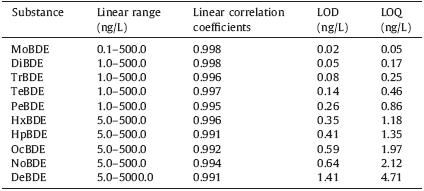
Qualitative analysis took at least two particular fragments of the PBDE standards. Quantitative analysis was carried out by external calibration using PBDE standard solutions spiked with 2.0 ng/L of dibromobiphenyl as an internal standard (Fig. 5B). Quantification analysis of PBDEs was based on the peak areas of the ion peaks. Usage of the fragments of PBDEs for qualitative and quantitative analysis was listed in Table 1. Calibration curves were established throughout the range of 0.1-500.0 ng/L except DeBDE. For the DeBDE,the quantitative analysis was based on the ion fragment of m/z = 799 in the range of 1.0-5000.0 ng/L due to its facile degradation. The limit of detection (LODs) was obtained by injecting a standard mixture,and was calculated with the signalto- noise ratio (S/N) ≥3 in the SIM mode. The LODs of 9 PBDEs depended on the type of PBDEs. Nine of the calibration curves were linear within the given concentration ranges (0.1,1,10,50,250, 500 ng/L) with obtained linear correlation coefficients over 0.991 as shown in Table 2. Calibration curves of DeBDE were in the range of (1.0,50.0,100.0,500.0,2000.0,5000.0 ng/L). LODs and LOQs for PBDEs in water were in the range of 0.05-2.12 ng/L as shown in Table 2,while that of DeBDE was 4.71 ng/L.
|
Download:
|
| Fig. 5.The detection of PBDEs in water sample. (A) The behavior of PBDEs in the river water. (B) The TIC of internal standard dibromobiphenyl with 2 ng/L was analyzed in the water sample. (C) The TIC of PeBDE. (D) The TIC of OcBDE. (E) The SIM of OcBDE. (a) Total ion chromatogram of PBDE with dibromobiphenyl standard solution. (b) Total ion chromatogram of PBDE with dibromobiphenyl spiked into water sample. | |
| Table 2 The linearity,LOD and LOQ for PBDEs assay. |
LLE-GC-MS was applied for the determination of PBDEs in the three river water samples. The samples were collected and immediately analyzed as described above. Fig. 5B-D showed the chromatogram of a river water sample spiked with 2 ng/L PBDE. Little matrix effects were observed,and LLE with mixed petroleum ether (60-90 8C) and hexane was selected as a good and reliable solvent to extract PBDEs from river water. Based on the retention times and the qualitative fragment ions,Fig. 5C and D showed that only PeBDE and OcBDE can be observed from river water samples. Interestingly,concentration of PeBDE was much lower than that of OcBDE. Then,Fig. 5E showed the fragments of OcBDE from river waters. Considering the fact that BFRs are used in the present study,we speculated that part of OcBDE came from the degradation of DeBDE and NoBDE,which also was confirmed by the SIM results. The high-level of OcBDE may be derived from following aspects. First,DeBDE was the chief brominated diphenyl ethers allowed to be used now,and DeBDE could easily be degraded to OcBDE. This process led to the higher concentration of OcBDE in river water. Second,compared with other highly brominated diphenyl ethers,such as NoBDE,DeBDE and HpBDE, OcBDE was more difficult to degrade in environment,resulting in OcBDE accumulation in river water. Furthermore,a great deal of OcBDE was used in the last decade,which may have resulted in more OcBDE released to the environment.
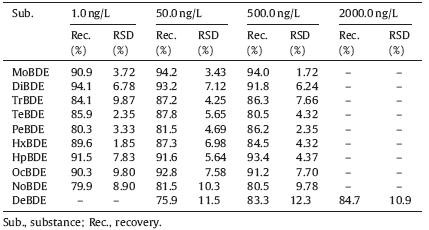
To ensure the accuracy of the results,recovery study was carried out by spiking the standard PBDEs solution into river water, and was performed in triplicate. The assay was evaluated by analyzing water spiked with 1,50 and 500 ng/L of PBDEs (except DeBDE). As presented in Table 3,the recovery was between 79.9% and 94.2% and the precision ranged from 1.72% to 10.3%. The recovery of the proposed method is comparable to that of the DLLME assay and other methods such as SPE and SPME [23, 26]. For DeBDE,the recovery with 50.0,500.0 and 2000.0 ng/L were 75.9%, 83.3%,and 84.7%,respectively. The suitable data of recovery and precision suggested that the matrix effect was minimal,and LLEGC- MS can be employed to determine PBDEs in water.
| Table 3 Recoveries and RSDs for PBDEs assay. |
The proposed method based on LLE and silica gel chromatography was effective for extracting and determining PBDEs in river water. The extracted solutions were successfully applied to GC-MS with high separation efficiency using capillary columns. Importantly, this approach wasstable and highly accurate. By carrying out a recovery study of real samples,the developed method was proven to be simple and reliable. Finally,the proposed methodology was successfully applied for the analysis of PBDEs in water samples. Acknowledgments
This work was supported by the Research Fund for the Doctoral Program of Higher Education (No. 20110002110052) and China Equipment and Education Resources System (No. CERS-1-75).
| [1] | N. Kajiwara, H. Takigami, Emission behavior of hexabromocyclododecanes and polybrominated diphenyl ethers from flame-retardant-treated textiles, Environ. Sci. 15 (2013) 1957-1963. |
| [2] | S. Siddique, Q.M. Xian, N. Abdelouahab, et al., Levels of dechlorane plus and polybrominated diphenylethers in human milk in two Canadian cities, Environ. Int. 39 (2012) 50-55. |
| [3] | J. Muñoz-Arnanz, M. Sáez, J.I. Aguirre, et al., Predominance of BDE-209 and other higher brominated diphenyl ethers in eggs of white stork (Ciconia ciconia) colonies from Spain, Environ. Int. 37 (2011) 572-576. |
| [4] | Y. Kang, H.S. Wang, K.C. Cheung, M.H. Wong, Polybrominated diphenyl ethers (PBDEs) in indoor dust and human hair, Atmos. Environ. 45 (2011) 2386-2393. |
| [5] | Y. Li, T.Wang,Y. Hashi, H. Li, J.M. Lin, Determination of brominatedflameretardants in electrical and electronic equipments with microwave-assisted extraction and gas chromatography-mass spectrometry, Talanta 78 (2009) 1429-1435. |
| [6] | J.P. Boon, W.E. Lewis, M.R. Tjoen-A-Choy, et al., Levels of polybrominated diphenyl ether (PBDE) flame retardants in animals representing different trophic levels of the North Sea food web, Environ. Sci. Technol. 36 (2002) 4025-4032. |
| [7] | A. Besis, C. Samara, Polybrominated diphenyl ethers (PBDEs) in the indoor and outdoor environments -a review on occurrence and human exposure, Environ. Pollut. 169 (2012) 217-229. |
| [8] | A. Covaci, S. Harrad, M.A.-E. Abdallah, et al., Novel brominated flame retardants: a review of their analysis, environmental fate and behaviour, Environ. Int. 37 (2011) 532-556. |
| [9] | X. Lin, H.F. Li, X. He, et al., Automated online pretreatment and cleanup recycle coupled with high-performance liquid chromatography-mass spectrometry for determination of deca-bromodiphenyl ether in human serum, J. Sep. Sci. 35 (2012) 2553-2558. |
| [10] | J.L. Domingo, Polybrominated diphenyl ethers in food and human dietary exposure: a review of the recent scientific literature, Food Chem. Toxicol. 50 (2012) 238-249. |
| [11] | L.G. Costa, R. de Laat, S. Tagliaferri, C. Pellacani, A mechanistic view of polybrominated diphenyl ether (PBDE) developmental neurotoxicity, Toxicol. Lett. 378 (2013) 1413-1417. |
| [12] | N. Xiang, X. Zhao, X.Z. Meng, L. Chen, Polybrominated diphenyl ethers (PBDEs) in a conventional wastewater treatment plant (WWTP) from Shanghai, the Yangtze River Delta: implication for input source and mass loading, Sci. Total Environ. 461-462 (2013) 391-396. |
| [13] | H.B. Moon, M. Choi, J. Yu, R.H. Jung, H.G. Choi, Contamination and potential sources of polybrominated diphenyl ethers (PBDEs) in water and sediment from the artificial Lake Shihwa, Korea, Chemosphere 88 (2012) 837-843. |
| [14] | Y. Wang, X. Li, A. Li, et al., Effect of municipal sewage treatment plant effluent on bioaccumulation of polychlorinated biphenyls and polybrominated diphenyl ethers in the recipient water, Environ. Sci. Technol. 41 (2007) 6026-6032. |
| [15] | I. Ali, V. Gupta, Advances in water treatment by adsorption technology, Nat. Prot. 1 (2007) 2661-2667. |
| [16] | S. Rayne, M.G. Ikonomou, Polybrominated diphenyl ethers in an advanced wastewater treatment plant. Part 1: Concentrations, patterns, and influence of treatment processes, J. Environ. Eng. Sci. 4 (2005) 353-367. |
| [17] | L. Fang, J. Huang, G. Yu, L. Wang, Photochemical degradation of six polybrominated diphenyl ether congeners under ultraviolet irradiation in hexane, Chemosphere 71 (2008) 258-267. |
| [18] | A.P. Vonderheide, K.E. Mueller, J. Meija, G.L. Welsh, Polybrominated diphenyl ethers: causes for concern and knowledge gaps regarding environmental distribution, fate and toxicity, Sci. Total Environ. 400 (2008) 425-436. |
| [19] | Y.T. Guo, H.B. Wu, G.H. Wang, et al., Progress on biodegradation of polybrominated diphenyl ethers by microorganisms, Environ. Sci. Technol. 3 (2012) 030. |
| [20] | Y. Li, Y. Hashi, Y. Liu, H.F. Li, J.M. Lin, Comparison and optimization of several pretreatment techniques for determination of decabrominated diphenyl ether in polymer sample by gas chromatography-mass spectrometry, Anal. Sci. 25 (2009) 523-527. |
| [21] | D. Ueno, C. Darling, M. Alaee, et al., Hydroxylated polybrominated diphenyl ethers (OH-PBDEs) in the abiotic environment: surface water and precipitation from Ontario, Canada, Environ. Sci. Technol. 42 (2008) 1657-1664. |
| [22] | M. Rezaee, Y. Yamini, M. Faraji, Evolution of dispersive liquid-liquid microextraction method, J. Chromatogr. A 1217 (2010) 2342-2357. |
| [23] | Y. Li, J. Hu, X. Liu, et al., Dispersive liquid-liquid microextraction followed by reversed phase HPLC for the determination of decabrominated diphenyl ether in natural water, J. Sep. Sci. 31 (2008) 2371-2376. |
| [24] | N. Fontanals, T. Barri, S. Bergström, J.Å. Jönsson, Determination of polybrominated diphenyl ethers at trace levels in environmental waters using hollow-fiber microporous membrane liquid-liquid extraction and gas chromatography-mass spectrometry, J. Chromatogr. A 1133 (2006) 41-48. |
| [25] | H. Liu, Q. Zhang, Z. Cai, et al., Separation of polybrominated diphenyl ethers, polychlorinated biphenyls, polychlorinated dibenzo-dibenzo-p-dioxins and dibenzo-furans in environmental samples using silica gel and florisil fractionation chromatography, Anal. Chim. Acta 557 (2006) 314-320. |
| [26] | X. Liu, J. Li, Z. Zhao, et al., Solid-phase extraction combined with dispersive liquid-liquid microextraction for the determination for polybrominated diphenyl ethers in different environmental matrices, J. Chromatogr. A 1216 (2009) 2220-2226. |
| [27] | S.I. Kawano, Y. Inohana, Y. Hashi, J.M. Lin, Analysis of keto-enoltautomers of curcumin by liquid chromatography/mass spectrometry, Chin. Chem. Lett. 24 (2013) 685-687. |
| [28] | S.S. Liu, Y. Liu, D.Q. Yin, L.S. Wang, Predicting the relative retention time (RRT) of polybrominated diphenyl ethers (PBDEs), Chin. Chem. Lett. 16 (2005) 1559-1662. |
| [29] | G.J. Wang, M.L. Qi, Analysis of volatile compounds in Herba asari by single-drop micro-extraction gas chromatography-mass spectrometry, Chin. Chem. Lett. 24 (2013) 542-544. |