Jin-Yin-Hua,the flower buds of Lonicera japonica Thunb. (Caprifoliaceae),also known as honeysuckle,is one of the most common ingredients of formulations used in traditional Chinese medicine for treating influenza,colds,fevers,and infections [1]. Chemical and pharmacological studies have resulted in characterization of constituents with different structural features and biological activities fromextracts of thismedicine,including caffeoyl quinic acids,secoiridoids,flavonoids,saponins,cerebrosides,polyphenols, and nitrogen containing iridoids [2, 3, 4, 5, 6, 7, 8, 9, 10, 11, 12, 13]. As part of a programtoassess the chemical and biological diversity of traditional Chinese medicines,we conducted detailed chemical analysis of an aqueous extract of the flowerbuds of L. japonica,since the flowerbud decoction is practically used in the formulations. Our previous studies on the aqueous extract led to the isolation of 27 homosecoiridoids having structural characters of the secoiridoid nucleus coupled with N-substituted nicotinic acid or pyridine units (lonijaposides A-W),and the secoiridoid nucleus coupled with phenylpyruvic acid derived moieties (loniphenyruviridosides A-D) [14, 15, 16]. In addition,after the flower buds were extracted bywater, the residue was further extracted with EtOH (95%),from which six new aromatic glycosides and 48 known compounds were characterized [17, 18]. Some of these compounds showed antiviral activity against the influenza virus A/Hanfang/359/95 (H3N2) and Coxsackie virus B3 replication,as well as anti-inflammatory activity against the release of glucuronidase in rat polymorphonuclear leukocytes induced by the platelet-activating factor and STAT-3 (signal transducers and activators of transcription 3) inhibitory activity. In continuing investigations on the aqueous extract,two uncommon β-hydroxy amino acid-coupled secoiridoids 1 and 2 have been characterized. We report herein the isolation,structure elucidation,semisynthesis,and biological activities of these two isolates. 2. Experimental 2.1. General experimental procedures
Optical rotations were measured on a PE Model 343. UV spectra were measured on a JASCO J-810 spectropolarimeter. IR spectra were recorded on a Nicolet 5700 FT-IR Microscope spectrometer (FT-IR Microscope Transmission). 1D- and 2D-NMR spectra were obtained at 500 MHz or 600 MHz for 1H,and 125 MHz or 150 MHz for 13C,respectively,on INOVA 500 MHz or SYS 600 MHz spectrometers with solvent peaks as references (unless otherwise noted). FABMS and HR-FABMS data were measured on a Micromass Auto spec-Ultima ETOF spectrometer. ESIMS data were measured with a Q-Trap LC/MS/MS (Turbo Ionspray source) spectrometer. HR-ESIMS data were,in turn,measured on an AccuToFCS JMS-T100CS spectrometer. Column chromatography was performed with silica gel (200-300 mesh,Qingdao Marine Chemical Inc.,Qingdao,China) and Pharmadex LH-20 (Pharmacia Biotech AB,Uppsala,Sweden). HPLC separation was performed on an instrument with a Waters 600 controller,a Waters 600 pump, and a Waters 2487 dual l absorbance detector on a Prevail (250 × 10 mm i.d.) semi-preparative column packed with C18 (5 mm). Glass precoated silica gel GF254 plates were used for TLC. Spots were visualized under UV light or by spraying with 7% H2SO4 in 95% EtOH followed by heating. 2.2. Plant material
See Ref. [14]. 2.3. Extraction and isolation
For extraction and preliminary fractionation of the extract,see Refs. [14, 15]. Fraction B2 (8.4 g) was separated by flash chromatography over RP silica gel,eluting with a gradient of EtOH (0- 100%) in H2O to give subfractions (B2-1-B2-20). B2-11 (1.2 g) was subjected to RP-HPLC,using CH3CN-H2O (12:88) containing 0.1% HOAc as the mobile phase,to afford fractions B2-11-1-B2-11-8. B2-11-6 (68 mg) was purified by RP-HPLC,using CH3CN-H2O (16:84) containing 0.1% HOAc as the mobile phase,to yield 2 (8.9 mg,0.00007%). Fraction B4 (86 g) was chromatographed over a RP silica gel column,eluting with a gradient of EtOH (0-100%) in H2O,to yield subfractions B4-1-B4-7,of which subfraction B4-7 (1.4 g) was further separated by flash chromatography over RP silica gel,eluting with a gradient of MeOH (0-50%) in H2O to give subfractions B4-7-1-B4-7-4. B4-7-4 (108 mg) was subjected to RPHPLC, using CH3OH-H2O (6:4) containing 0.1% HOAc as the mobile phase,to afford 1 (59.3 mg,0.00049%).
Serinosecologanin (1): White amorphous powder,soluble in H2O,MeOH,and EtOH; [α]D20 -183.4 (c 0.40,H2O); UV (H2O) λmax (log e) 241.0 (4.06) nm; IR vmax 3326,2883,1720 (sh),1657,1584, 1465,1400,1358,1314,1255,1210,1168,1056,1014,913,887, 837,751,713,693,633 cm-1; 1HNMR(D2O,600 MHz),see Table 1; 13C NMR (D2O,150 MHz),see Table 1; (+)-FABMS m/z 444 [M+H]+, 466 [M+Na]+,482 [M+K]+; HR-ESIMS m/z 444.1512 [M+H]+ (calcd. for C19H26NO11 444.1506).
Threninosecologanin (2): White amorphous powder,soluble in H2O,MeOH,and EtOH; [α]D20 -156.8 (c 0.37,H2O); UV (H2O) lmax (log e) 240 (4.36) nm; IR nmax 3386,2935,1722,1656,1589,1560, 1399,1341,1310,1207,1170,1121,1064,1014,955,899,840, 771,751,694 cm-1; 1H NMR (D2O,500 MHz),see Table 1; 13C NMR (D2O,125 MHz),see Table 1; (+)-ESIMS m/z 458 [M+H]+,480 [M+Na]+,496 [M+K]+; (-)-ESIMS m/z 456 [M-H]-,491 [M+Cl]-; HR-ESIMS m/z 480.1494 [M+Na]+ (calcd. for C20H27NO11Na 480.1482).
| Table 1 NMR spectroscopic data for compounds 1 and 2.a |
Each compound (~1 mg) in H2O (~1 mL) was treated with bglucosidase from almonds (10 mg,8.92 U/mg,Mw135000,SigmaAldrich Corporation,USA),hesperidinase from Aspergillus niger (10 mg,3 U/g,Sigma-Aldrich),or snailase (5 mg,Shanghai Sangon Biotech Co.,Ltd.,China) at 37 ℃ for 20-96 h. Thin layer chromatography (TLC,CHCl3-MeOH-HOAc 3:1:0.3) detection indicated that 1 and 2 were not hydrolyzed by β-glucosidase and hesperidinase,but disappeared on hydrolysis with snailase. Then,an aqueous solution (1 mL) of each compound (5 mg) was treated with snailase (20 mg) at 37 ℃ for 12 h. The reaction mixtures were extracted with EtOAc (2 × 3 mL). The H2O phases were separately concentrated to dryness,and the residues were chromatographed over silica gel,eluting with CH3CN-H2O (6:1),to yield glucose with [α]D20 values of +47.3 (c 0.19,H2O) and +43.1 (c 0.07,H2O) from the hydrolysates of 1 and 2. The solvent system CH3CN-H2O (4:1) was used for TLC identification of glucose (Rf = 0.39). 2.5. Determination of the absolute configurations of amino acid units in 1 and 2
Compounds 1 (1.0 mg) and 2 (0.7 mg) were separately hydrolyzed with 6 mol/L HCl (200 μL) in a sealed glass bomb at 110 ℃ for 16 h. The solutions were evaporated in vacuo. To the residues,FDAA [(1-fluoro-2,4-dinitrophenyl)-5-L-alanine amide] solution in acetone (1%,300 mL) and 6% aqueous triethylamine (150 μL,1 mol/L) were added. The mixture was stirred at 40 ℃ for 1 h,then diluted with H2O (500 μL),and filtered. The standard FDAA-amino acids were prepared in the same way,using L-serine, D-serine,L-threonine,and D-threonine (1.1-1.3 mg),respectively. The FDAA-amino acid derivatives from the hydrolysate were compared with the standard FDAA-amino acids by HPLC analysis: Alltech Alltima C18 column (250 × 4.6,5 μm),flow rate 1 mL/min, UV detection at 340 nm,mobile phase CH3CN-H2O (18:82) containing 1% AcOH. The retention times tR,are as follows: FDAA derivative of the hydrolysate from 1,24.2 min; FDAA derivative of the hydrolysate from 2,32.4 min; FDAA-L-serine,24.1 min; FDAAD- serine,26.5 min; FDAA-L-threonine,32.4 min; and FDAA-Dthreonine, 43.6 min. 2.6. Synthesis of 1 and 2
Secologanin (3) or secologanic acid (4) (30-50 mg) was refluxed with two molar equivalents of L-serine or L-threonine in acetonitrile or pyridine for 30-50 h,respectively. The reaction mixtures were evaporated to give corresponding residues which were separately isolated by preparative thin layer chromatography (PTLC) over silica gel,eluting with the mobile phase CHCl3-MeOH- HOAc (3:1:0.3),to afford 1 and 2 (64-93% yields) from the reactions of 3 or 4 with L-serine and L-threonine,respectively. The 1H NMR,ESIMS,and [α]D20 data of 1 and 2 were consistent with those of the natural products. 2.7. Determination of β-glucosidase resistance of 1 and 2
Standard solutions (10 mmol/L) of 1,2,and sweroside,and a solution (0.66 mg/μL) of β-glucosidase (from almonds) were prepared with a KH2PO4/K2HPO4 buffer (pH 6.8) [19]. A mixture of the standard solutions of sweroside (100 μL) and enzyme (100 μL),as well as a mixture of the standard solutions of sweroside (100 μL),enzyme (100 μL),and 1 (100 mL) or 2 (100 mL) were incubated at 37 ℃. After incubation for 10,30,and/or 60 min, the mixtures were analyzed by RP-HPLC using an Altech Brava C18 column (250 mm × 4.6 mm,i.d.,5 μm) and mobile phase CH3CN- H2O (15:85) containing 0.1% HOAc. The chromatograms indicated that 1 and 2 were not hydrolyzed by β-glucosidase,whereas sweroside was hydrolyzed. In addition,the hydrolysis of sweroside with the enzyme was not disturbed by the presence of 1 and 2. 2.8. Assays for pharmacological activities of 1 and 2
Details may be found in Refs. [14, 15, 16] and the references cited therein. 3. Results and discussion
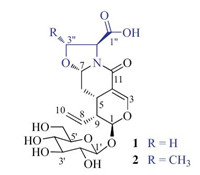
Compound 1 was obtained as a white amorphous solid,[α]D20 -183.4 (c 0.40,H2O). Its IR spectrum showed absorption bands for hydroxy (3326 cm-1) and carbonyl [1720 (sh),1657,and 1584 cm-1] functionalities. The positive FABMS of 1 exhibited pseudo molecular ion peaks at m/z 444 [M+H]+,466 [M+Na]+,and 482 [M+K]+. HRFABMS at m/z 444.1512 [M+H]+ (calcd. for C19H26NO11,444.1506) indicated the molecular formula C19H25NO11,which was supported by the NMR data (Table 1). The 1H NMR spectrum of 1 in D2O showed signals attributed to a trisubstituted olefinic proton at δH 7.47 (brs,H-3),two acetal protons at δH 5.58 (d,J = 1.8 Hz,H-1) and 5.13 (dd,J = 9.6 and 3.6 Hz,H-7),and a monosubstituted vinyl group at δH 5.50 (ddd, J = 16.8,10.2,and 10.2 Hz,H-8),5.39 (dd,J = 16.8 and 1.2 Hz,H- 10b),and 5.31 (d,J = 10.2 and 1.2 Hz,H-10b). In addition,the spectrum showed resonances due to two methines at δH 3.10 (J = 13.2,6.0,and 3.6 Hz,H-5) and 2.85 (J = 10.2,6.0,and 1.8 Hz,H- 9) and a methylene at δH 1.43 (ddd,J = 13.2,12.0,and 9.6 Hz,H-6a) and 2.31 (ddd,J = 12.0,3.6,and 3.6 Hz,H-6b). Characteristic signals due to a β-glucopyranosyl unit were observed at δH 4.86 (d, J = 8.4 Hz,H-10),3.31 (dd,J = 9.6 and 8.4 Hz,H-20),3.52 (t,J = 9.6 Hz, H-30),3.42 (t,J = 9.6 Hz,H-40),3.54 (m,H-50),3.94 (dd,J = 12.6 and 1.8 Hz,H-60a),and 3.75 (dd,J = 12.6 and 6.0 Hz,H-60b). Also present was an ABX coupling system assignable to a serine unit at δH 4.58 (dd,J = 9.0 and 8.4 Hz,H-300a),3.95 (dd,J = 9.0 and 8.4 Hz, H-300b),and 4.71 (t,J = 8.4 Hz,H-200). Besides the resonances corresponding to the above protonated carbons (Table 1),the 13C NMR and DEPT spectra of 1 showed resonances for two carbonyl carbons at dC 176.6 (C-100) and 167.2 (C-11),and a quaternary olefinic carbon at dC 109.6 (C-4). These spectroscopic data suggest that 1 is an unusual secoiridoid glycoside [14, 15, 16] containing a serine unit. The suggestion was confirmed by comprehensive analysis of the 2D NMR data,which resulted in an unambiguous structure determination of 1.
The proton and protonated carbon signals in the NMR spectra of 1 were unequivocally assigned by interpreting the 1H-1HCOSY and HMQC spectroscopic data. The 1H-1H COSY spectrum of1 displayed homonuclear vicinal coupling correlations: H-1/H-9/ H-5/H2-6/H-7 and H-9/H-8/H2-10,in addition to W-type correlations H-1/H-3/H-5 (Fig. 2,thick lines). These,combined with twoand three-bond correlations in the HMBC spectrum (Fig. 2,red arrows): H-1/C-3,C-5,C-8,and C-9; H-3/C-1,C-4,C-5,and C-11; H- 5/C-1,C-3,C-4,C-6,C-7,C-8,C-9,and C-11; H2-6/C-4,C-5,C-7,and C-9; H-7/C-5; H-8/C-1,C-5,C-9,and C-10; H-9/C-1,C-4,C-5,C-6,C- 8,and C-10; and H2-10/C-8 and C-9; as well as the chemical shifts of these proton and carbon resonances,revealed unambiguously the presence of a secoiridoid parent nucleus with 7-acetalic and 8(10)-olefinic functionalities in 1. The COSY correlations H-10/H-20/ H-30/H-40/H-50/H2-60 and the HMBC correlations H-1/C-10 and H- 10/C-1 verified the presence of a β-glucopyranosyl moiety at C-1 of the nucleus. Additionally,the COSY correlations H-2''/H2-3'' and the HMBC correlations from H-2'' to C-100,C-3'',C-7,and C-11 and from H2-3'' to C-1'' and C-7,together with their chemical shifts, demonstrated the presence of the serine unit,of which C-2'' and C- 3'' were connected through nitrogen and oxygen atoms to C-7 of the nucleus. The HMBC correlation from H-7 to C-11,combined with the molecular composition,indicated that the carbonyl carbon (C-11) was linked through the nitrogen atom to C-7 to form a lactam in 1. Accordingly,the planar structure of compound 1 was elucidated as shown in Fig. 2.
In the NOE difference spectrum,irradiation of H-5 enhanced the H-6β,H-7,and H-9 resonances,while the H-5,H-6β,and H-3''β resonances were enhanced upon irradiation of H-7 (Fig. 3,red dashed lines). The enhancements revealed that H-5,H-6β,H-7,H- 9,and H-3''β were cofacial on one side of the ring system. The enhancements of the H-1,H-8 and H2-10 resonances upon irradiation of H-6a indicated that these protons were cofacial on the other side of the ring system. This suggested that the secoiridoid nucleus in 1,with a β-oriented H-7,had the same configuration as that of the co-occurring secologanic acid,for which the absolute configuration was determined by a singlecrystal X-ray crystallographic analysis using anomalous scattering of Cu Ka radiation [15]. In addition,the H-2'' resonance was not enhanced by irradiation of H-7,and,in turn,irradiation of H-200 did not enhanced the H-7 resonance. This suggests that H-7 is opposite H-200 on the oxazolidine ring in 1. Acid hydrolysis of 1,followed by Marfey’s analysis of the hydrolysate [20],revealed a production of L-serine on hydrolysis. This supports a trans-orientation of H-7 and H-200. Enzymatic hydrolysis of 1 with snailase,produced glucose identified on the basis of TLC by comparison with an authentic sugar sample. The glucose isolated from the hydrolysate gave a positive optical rotation,[α]D20 +47.3 (c 0.19,H2O),indicating that it was D-glucose [14, 15]. Based on the enzymatic and acidic hydrolyses and the aforementioned NOE enhancements,the absolute configuration of 1 was assigned as shown in Fig. 1. Therefore,the structure of 1 was determined and the compound was designated as serinosecologanin.
|
Download:
|
| Fig. 1.Structures of compounds 1 and 2. | |
Compound 2 was obtained as a white amorphous solid,[α]D20 - 156.8 (c 0.37,H2O). The positive ESIMS exhibited pseudo molecular ion peaks at m/z 458 [M+H]+,480 [M+Na]+,and 496 [M+K]+. The molecular formula C20H27NO11,with one CH2 unit more than that of 1,was indicated by HR-ESIMS at m/z 480.1494 [M+Na]+ (calcd. for C20H27NO11Na 480.1482). The UV,IR and NMR spectroscopic features of 2 were similar to those of 1. Comparing the NMR data between 2 and 1 (Table 1) indicated replacement of the serine unit in 1 by a threonine moiety in 2. This was confirmed by 2D NMR data analysis of 2. In particular,the 1H-1H COSY correlations H-200/ H-3''/H3-4'' and the HMBC correlations H-2''/C-1'',C-3'',and C-4''; H-3''/C-100; H20-4''/C-2'' and C-3'' (Fig. 2),in combination with their shifts,proved the presence of the threonine unit in 2. Additionally, the HMBC correlations H-2''/C-7 and C-11 verified the connection of C-7 and C-11 of the secoiridoid moiety to the nitrogen atom of the threonine unit. Although the correlations H-3''/C-7 andH-7/C-3'' were not observed in the HMBC spectrum of 2 due to the limited amount of sample,the chemical shift of C-7 (δC 90.1),similar to that of 1,supports the connection between the threonine oxygen atom and C-7. In the NOE difference spectrum of 2,irradiation of H-2'' enhancedtheH3-400 resonance,andtheH-7resonancewas enhanced by irradiation of H-3'' (Fig. 3). In turn,when H-7 was irradiated,the H-3'',H-5,and H-6β resonances were enhanced. These enhancements not only indicated that 2 possessed the configuration shown in Fig. 1,but also confirmed the oxygen-bridged connection between C-300 and C-7. The absolute configuration of 2 was verified by the isolation of D-glucose {[α]D20 +43.1 (c 0.07,H2O)} fromthe enzymatic hydrolysate and by Marfey’s analysis indicating release of Lthreonine from the acid hydrolysate. Therefore,the structure of compound 2 was determined and named threoninosecologanin.

|
Download:
|
| Fig. 2.Main 1H-1H COSY (black thick lines) and HMBC correlations (red arrows, from 1H to 13C) of 1 and 2. | |

|
Download:
|
| Fig. 3.NOEenhancements (red dash arrows) in theNOEdifference spectra of 1 and 2. | |
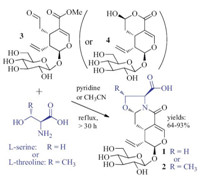
Secologanin (3) is proposed to be the biogenetic precursor of about 2000 alkaloids and other bioactive natural products,such as reserpine,vincristine,and camptothecin [21],and has been extensively used in synthetic and biosynthetic studies of monoterpene alkaloids and relatednatural products. This indicates that1 and 2 could be biosynthesized from the co-occurring secologanin (3) and/or secologanic acid (4) [14, 15]. To provide chemical support to this proposition,confirm the absolute configurations,and assay their biological activities,compounds 1 and 2 were synthesized by refluxing 3 or 4 with L-serine or L-threonine in acetonitrile or pyridine for 30-50 h,respectively (Scheme 1). Interestingly,the reactions did not take place in acetonitrile or pyridine at 20 ℃ even after 24 h [21]. The physical-chemical properties of the synthetic 1 and 2 were identical with those of the natural products. Because a single diastereomer of 1 or 2 was generated in the reaction,with H-7 having the axial b orientation as shownby the splitting patterns and coupling constants (J6α,7β = 9.6 Hz and J6β,7β = 3.6 Hz),the stereo-selectivity in the formation of 1 or 2 was similar to that in the condensation of 3 with functionalized amines. This indicates a similar stereo-controlled mechanism for the reaction [21].
|
Download:
|
| Scheme 1.Synthesis of compounds 1 and 2. | |
In the enzymatic hydrolyses of compounds 1 and 2,neither compound could be hydrolyzed by common enzymes of plant origin (β-glucosidase from almonds and hesperidinase from A. niger),but could be hydrolyzed by snailase,of animal origin. In contrast,other analogs from the same materials [14, 15, 16],such as 3, 4,and sweroside (5) can readily be hydrolyzed by all the enzymes. Thus,resistance and/or inhibitory activities of 1 and 2 against bglucosidase were preliminarily investigated. Compound 5,without or with 1 or 2,was hydrolyzed using β-glucosidase (Scheme 2). HPLC analysis revealed that time-dependent hydrolysis of 5 was not disturbed by the presence of 1 or 2 in the hydrolytic system, although 1 or 2 was not hydrolyzed (Figs. S27,S28,and S29 in Supporting information). This indicates that 1 and 2 are not inhibitors of the enzyme and they are resistant to the bglucosidase. Therefore,these unusual structures are not substrates of the enzyme.

|
Download:
|
| Scheme 2. Hydrolysis of sweroside (5). | |
In preliminary in vitro assays,compounds 1 and 2,at 10 mmol/L, showed inhibitory activity against the release of glucuronidase in rat polymorphonuclear leukocytes induced by the plateletactivating factor,with inhibition rates of (34.9 ± 3.1)% and 53.6 ± 2.6%,respectively,while the positive control (ginkgolide B) exhibited an inhibition rate of (73.0 ± 3.3)% at the same concentration [14]. The protective activities of the compounds against neurotoxicity induced by serum deprivation in PCl2 cells were investigated by the MTT method. The results showed that serum deprivation induced significant inhibition of MTT reduction,at a concentration of 10 mmol/L. Compounds 1 and 2 showed cell viability from (71.0 ± 2.4)% (control) to (77.5 ± 3.0)% and (78.9 ± 1.6)%, respectively,indicating that 2 may be effective in neurodegenerative disorders. Compounds 1 and 2 were also assessed for their activities against influenza virus A/Hanfang/359/95 (H3N2),Coxsackie virus B3, and HIV-1 replication,as well as against several human cancer cell lines [16],but all were inactive at a concentration of 10 mmol/L. 4. Conclusion
Two β-hydroxy amino acid-coupled secoiridoids,serinosecologanin (1) and threoninosecologanin (2) were isolated from an aqueous extract of the flower buds of L. japonica. Their unique structures,including their absolute configurations,were elucidated by spectroscopic data analysis and synthesis. Compounds 1 and 2 exhibited activity against the release of glucuronidase in rat polymorphonuclear leukocytes,induced by the platelet-activating factor,as well as resistance against β-glucosidase fromalmonds and hesperidinase from A. niger. This is the first example of natural iridoid glycosides resistant to the β-glucosidases of plant origin. These results,combined with our previous studies [14, 15, 16, 17, 18],suggest that the diverse constituents have pharmacological contributions to the traditional uses of theflowerbuds of L. japonica. Semisynthesis of 1 and 2 fromthe co-occurring abundant analogs secologanin (3) and/ or secologanic acid (4),not only supports their biosynthetic relationship,but also provides an important approach to synthesize and modify the structures of the minor constituents for further investigation of their biological activities and structure-activity relationships. Acknowledgments
Financial support from the National Natural Science Foundation of China (NNSFC; Nos. 20772156 and 30825044),the Program for Changjiang Scholars and Innovative Research Team in University (PCSIRT,No. IRT1007),and the National Science and Technology Project of China (Nos. 2012ZX09301002-002 and 2011ZX0 9307- 002-01) is acknowledged. Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.05.037.
| [1] | Jiangsu New Medical College, Dictionary of Traditional Chinese Medicine, Shanghai Science and Technology Publishing House, Shanghai, 1977, pp. 1403-1405. |
| [2] | R. Mehrotra, C. Singh, S.P. Popli, Isolation of secoxyloganin from Lonicera japonica and its conversion into secologanin, J. Nat. Prod. 51 (1988) 319-321. |
| [3] | H. Kawai, M. Kuroyanagi, A. Ueno, Iridoid glycosides from Lonicera japonica Thunb, Chem. Pharm. Bull. 36 (1988) 3664-3666. |
| [4] | K.H. Son, J.O. Park, K.C. Chung, et al., Flavonoids from the aerial parts of Lonicera japonica, Arch. Pharm. Res. 15 (1992) 365-370. |
| [5] | L. Tomassini, M.F. Cometa, M. Serafini, M. Nicoletti, Isolation of secoiridoid artifacts from Lonicera japonica, J. Nat. Prod. 58 (1995) 1756-1758. |
| [6] | R.W. Teng, D.Z. Wang, C.X. Chen, Two triterpenoid saponins from Lonicera japonica, Chin. Chem. Lett. 11 (2000) 337-340. |
| [7] | R. Kakuda, M. Imai, Y. Yaoita, et al., Secoiridoid glycosides from the flower buds of Lonicera japonica, Phytochemistry 55 (2000) 879-881. |
| [8] | C.W. Choi, H.A. Jung, S.S. Kang, et al., Antioxidant constituents and a new triterpenoid glycoside from Flos Lonicerae, Arch. Pharm. Res. 30 (2007) 1-7. |
| [9] | D.Q. Yu, R.Y. Chen, L.J. Huang, et al., The structure and absolute configuration of Shuangkangsu: a novel natural cyclic peroxide from Lonicera japonica (Thunb.), J. Asian Nat. Prod. Res. 10 (2008) 851-856. |
| [10] | L.M. Lin, X.G. Zhang, J.J. Zhu, et al., Two new triterpenoid saponins from the flowers and buds of Lonicera japonica, J. Asian Nat. Prod. Res. 10 (2008) 925-929. |
| [11] | C.Y. Chen, W.H. Peng, L.C. Wu, C.C. Wu, S.L. Hsu, Luteolin ameliorates experimental lung fibrosis both in vivo and in vitro: implications for therapy of lung fibrosis, J. Agric. Food Chem. 58 (2010) 11653-11661. |
| [12] | E.J. Lee, J.S. Kim, H.P. Kim, J.H. Lee, S.S. Kang, Phenolic constituents from the flower buds of Lonicera japonica and their 5-lipoxygenase inhibitory activities, Food Chem. 120 (2010) 134-139. |
| [13] | Z.F. Zheng, Q.J. Zhang, R.Y. Chen, et al., Four new N-contained iridoid glycosides from flower buds of Lonicera japonica, J. Asian Nat. Prod. Res. 14 (2012) 729-737. |
| [14] | W.X. Song, S. Li, S.J. Wang, et al., Pyridinium alkaloid-coupled secoiridoids from the flower buds of Lonicera japonica, J. Nat. Prod. 71 (2008) 922-925. |
| [15] | Y. Yu, W.X. Song, C.G. Zhu, et al., Homosecoiridoids from the flower buds of Lonicera japonica, J. Nat. Prod. 74 (2011) 2151-2160. |
| [16] | Y. Yu, C.G. Zhu, S.J. Wang, et al., Homosecoiridoid alkaloids with amino acid units from the flower buds of Lonicera japonica, J. Nat. Prod. 76 (2013) 2226-2233. |
| [17] | F. Wang, Y.P. Jiang, X.L. Wang, et al., Aromatic glycosides from the flower buds of Lonicera japonica, J. Asian Nat. Prod. Res. 15 (2013) 492-501. |
| [18] | F. Wang, Y.P. Jiang, X.L. Wang, et al., Chemical constituents from flower buds of Lonicera japonica, Chin. J. Chin. Mater. Med. 38 (2013) 1378-1385. |
| [19] | C. Bluchel, C.V. Ramana, A. Vasella, et al., Synthesis of monosaccharide-derived spirocyclic cyclopropylamines and their evaluation as glycosidase inhibitors, Helv. Chim. Acta 86 (2003) 2998-3036. |
| [20] | P. Marfey, Determination of D-amino acids II: use of a bifunctional reagent, 1,5-difluoro-2,4-dinitrobenzene, Carlsberg Res. Commun. 49 (1984) 591-596. |
| [21] | L.F. Tietze, C. Bä rtels, J. Fennen, Biomimetic synthesis of the monoterpene alkaloids xylostosidine and loxylostosidine A and of similar unnatural compounds by transformations of the monoterpene glycoside secologanin, Liebigs Ann. Chem. 1989 (1989) 1241-1245. |





