The detection of adenosine is of tremendous importance, because adenosine plays an important role in biochemical processes [1, 2, 3]. In 1995,an adenosine specific aptamer was isolated and then converted into fluorescent sensors using various strategies [4, 5, 6, 7]. However,most of these reported fluorescent aptamer sensors exhibited only moderate sensitivity because of its relatively low binding affinity (Kd = 5 mmol/L) [4]. Thus,a sensitive,fluorescent sensing design for the small biological molecule,adenosine,is highly desirable. Aptazyme,composed of aptamer and DNAzyme,is a specially engineered nucleic acid molecule that contains two functional regions: a ligand-binding motif that is responsive to a target of interest and a catalytic element that can carry out a chemical reaction under appropriate conditions. Usually,the aptazyme is designed in such a way that the function of its catalytic domain is dependent on the binding state of the aptamer element. However,most of those fluorescent aptazyme designs exhibited high background. Because the aptazyme and substrate are two different molecules,each of them is a labeled fluorophore or quencher [8, 9]. Thus,the intermolecular quenching efficiency would be slightly low. In order to solve this issue,a molecular beacon (MB) with high intramolecular quenching efficiency is introduced in our design. MBs are single-stranded oligonucleotides with a stem-loop structure that brings the quencher and fluorophore into close proximity,thereby fluorescence is quenched effectively [10, 11, 12]. Zhang et al. combined the DNAzyme and MB to develop a catalytic molecular beacon for the first time,which showed good sensitivity and selectivity [13, 14, 15]. Based on their work,we combined the aptazyme and MB to develop a catalytic molecular beacon for amplified detection of adenosine.
The strategy is based on the concept that the aptazyme activity could be regulated by the aptamer-target binding. If the aptamer binds to its target,the aptazyme would be active and then cleave the designed MB substrates at specific sites,where the substrate sequence is embedded in the loop of MB,resulting in stem dehybridization and an enhanced fluorescence signal. In this way, on one hand,the background is restrained by the MB with high intramolecular quenching efficiency. On the other hand,in the presence of excess MB substrates,one aptazyme can catalyze the cleavage of multiple MB substrates and achieve an amplified fluorescence signal. Thus,it is possible to achieve high sensitivity through suppressing the fluorescence background and elevating the fluorescence signal. 2. Experimental
Oligonucleotides used in this work were synthesized and purified by Takara Biotechnology Co.,Ltd. (Dalian,China),and their sequences are shown in Table 1. Adenosine and the analogs were purchased from Sigma-Aldrich Chemical Co.,Ltd. All work solutions were 50 mmol/L HEPES (200 mmol/L NaCl,pH 7.0). Fluorescence measurements were carried out on a Hitachi F-7000 fluorescence spectrometer (Hitachi. Ltd.,Japan). The excitation wavelength was set at 494 nm (slit 5 nm),and the emission spectra were collected from 510 nm to 650 nm (slit 5 nm). The fluorescence intensity at 521 nm was used to evaluate the performances of the proposed assay strategy. The pH measurements were carried out using an Orion 3 Star pH meter (Thermo Fisher,USA). All solutions were prepared with Milli-Q water (18.2 MV cm) from a Millipore system. For adenosine detection,different concentrations of adenosine were incubated with 100 nmol/L of In 4 (In denotes Inhibitor,see Table 1) in the buffer (50 mmol/L HEPES, 200 mmol/L NaCl,1 mmol/L MgCl2,1 mmol/L ZnCl2,pH 7.0) for 30 min at 25 ℃. Then,300 nmol/L MB were added into the above solution and incubated for another 2 h. Finally,the fluorescence emission intensity at 521 nm was measured.
| Table 1 Sequences of oligonucleotides used in this work. |
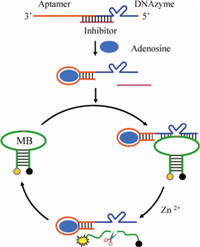
To illustrate the general design strategy of the inhibited aptazyme-based catalytic molecular beacon for amplified detection of adenosine,we chose a well-characterized adenosinespecific aptazyme which adapts 8-17 DNAzyme as the catalytic unit. The 8-17 DNAzyme is a small,RNA-cleaving DNA enzyme with high catalytic activity by adopting either Pb2+ or Zn2+ as cofactors [16, 17, 18]. To make an aptazyme whose activity can be regulated by aptamer-target binding,we simply attached the sequence of the adenosine aptamer (red line) to the 30-terminus of 8-17 DNAzyme (blue line) as illustrate in Scheme 1.
|
Download:
|
| Scheme 1.Schematic of the inhibited aptazyme-based catalytic molecular beacon for amplified detection of adenosine. | |
The enzymatic activity of the aptazyme is suppressed through the use of an oligonucleotide (purple line called inhibitor) that forms a stable DNA duplex spanning the aptamer-DNAzyme junction. In the absence of target adenosine,the inhibitor hybridizes to a portion of both the aptamer and DNAzyme,thus inhibiting the DNAzyme catalytic function. However,in the presence of target adenosine,the binding of the adenosine to the aptamer forces the dissociation of the inhibitor from the aptamer portion of the aptazyme,which significantly weakens the remaining duplex between the DNAzyme sequence and the inhibitor. As a result,the inhibitory effect of the inhibitor is much reduced and the DNAzyme catalytic function is active. Here,MB is employed to realize the true enzymatic multiple turnover of aptazyme and signal transduction. The DNAzyme substrate strand is incorporated in the loop of the MB (green line),which binds to the DNAzyme strand to form a complex structure. The active DNAzyme can cleave the substrate and cuts the MB into two pieces assisted by cofactors,resulting in stem dehybridization and enhanced fluorescence signal. In the presence of excess MB substrates,one DNAzyme can catalyze the cleavage of multiple MB substrates and achieve an amplified fluorescence signal.
Considering that DNA hybridization,aptamer-adenosine binding and DNAzyme catalytic efficiency were dominant factors in this method,a series of optimization experiments were performed to obtain a maximum signal change with and without target adenosine (F - F0). According to the literature [18],the concentrations of the divalent metal ions Zn2+ and Mg2+ were important for DNAzyme catalytic cleavage reactions and aptamer-adenosine binding,respectively. Investigative results of the two ions were presented in Fig. 1(a) and (b),the optimal concentrations of Zn2+ and Mg2+ were found to be 1 mmol/L. Meanwhile,a thermal profile of the method could be useful in determining the optimal binding and catalytic temperature. We obtained the thermal profiles of the method with or without target adenosine,characterized as the fluorescence intensity as a function of temperature. The optimal temperature for detection should be a temperature at which the fluorescence intensity gap (F - F0) reaches the maximum. As shown in Fig. 1(c),25 ℃ was chosen as the optimal temperature for the subsequent experiments.

|
Download:
|
| Fig. 1.Optimization of the inhibited aptazyme-based catalytic molecular beacon for amplified detection of adenosine. (a) Concentration of Zn2+; (b) concentration of Mg2+; (c) reaction temperature; (d) the molar ratio of the aptazyme to MB; (e) the different lengths of inhibitor; (f) the molar ratio of the aptazyme to inhibitor. F means the total fluorescence signal and F0 denotes fluorescence background. | |
Additionally,it is important to find an optimal ratio between the substrate strand MB and aptazyme so that the decrease of the quantity of the substrate induced by cleavage will be reflected in the fluorescence intensity change. If the substrate MB is insufficient, the catalytic efficiency of the 8-17 DNAzyme toward MB is restricted. Likewise,if the MB is in excess,only a small fraction of substrate can be cleaved at a set time. Both of them would not induce the optimal fluorescence signal,resulting in decreased sensitivity. To determine the optimal ratio of MB to aptazyme,we kept the aptazyme concentration at 100 nmol/L and then changed the concentrations of MB from 100 nmol/L to 1000 nmol/L. After incubation of 2 h at 25 ℃,the fluorescence enhancement was recorded. As shown in Fig. 1(d),the optimal ratio of MB to aptazyme is 3:1,which indicates that the active DNAzyme molecule could be regenerated after catalyzing the cleavage reaction so that one DNAzyme could cleave several MBs substrate molecules.
The conformation switching from aptazyme-inhibitor duplex to aptazyme-target complex was deemed to be dependent on the length of inhibitor. The inhibitor should insure the duplex is stable enough in the absence of target,but becomes unstable upon introduction of target. Different lengths of inhibitors (sequences shown from In 1 to In 7 in Table 1) were incubated with aptazyme and MB in the presence of 1 mmol/L adenosine and in the absence of adenosine for 2 h followed recording the fluorescence change. As seen in Fig. 1(e),we found the In 4 was appropriate sequence as inhibitor for this method. Finally,the ratio of aptazyme to inhibitor was also investigated. We kept the aptazyme concentration at 100 nmol/L and then changed the concentrations of In 4 from 100 nmol/L to 1000 nmol/L. The fluorescence result of Fig. 1(f) shows that the 1:1 ratio was optimal. In summary,the optimum detection condition was determined as follows: 100 nmol/L aptazyme,100 nmol/L In 4,300 nmol/L MB in the reaction buffer containing 1 mmol/L Zn2+ and 1 mmol/L Mg2+ at 25 ℃.
To provide a better understanding of the system,a kinetic assay of the aptazyme catalytic cleavage reaction was performed. We kept the above optimal condition and added 1 mmol/L adenosine, subsequently recorded the fluorescence signal every 10 min. Fig. 2(a) indicated that 30 min was needed for ~50% substrate cleavage and almost completely finished in 2 h. Thus,2 h was chosen as the assay time. Fig. 2(b) shows the adenosine-dependent fluorescence changes under the optimal procedure of inhibited aptazyme strategy. The target molecule adenosine can be quantified from 10 nmol/L to 1 mmol/L,with a detection limit of 10 nmol/L. To determine the selectivity of the system,1 mmol/L each of guanosine,cytidine,or uridine was used in the assay. Under the same condition,all the three nucleosides resulted in an insignificant fluorescence change. These results indicated that the high selectivity of the original aptazyme toward adenosine is maintained in this inhibited aptazyme strategy.
Since the adenosine aptamer was selected in 1995 [4], numerous aptamer-based adenosine assays have been developed using colorimetric,electrochemical,luminescent,and fluorescent signal output (see Table 2). However,the general low affinity of the adenosine aptamer,having a Kd of only 5 mmol/L,results in relatively high detection limits for these reported aptametric assays.
| Table 2 Comparison of aptamer-based adenosine sensors. |
In this paper,by combining the low background of the MB and the signal amplified function of DNAzyme,the inhibited aptazymebased catalytic molecular beacon for amplified detection of adenosine could drive the detection limit down to 10 nmol/L, which makes the sensitivity more than 1-3 orders of magnitude higher. 4. Conclusion
In summary,we have developed an inhibited aptazyme-based catalytic molecular beacon for amplified detection of adenosine with good sensitivity and selectivity. In comparison with previously reported aptamer-based probes,this new method has several advantages. First,the ratio of aptazyme and substrate is not limited to 1:1,which could be amplified by cycling and regeneration of the DNAzyme. Second,by utilizing the high intramolecular quenching efficiency of the molecular beacon,the background would be very low. Last but not least,by introduction of numerous aptamers into DNAzyme through an inhibition strategy,the aptazyme-based catalytic molecular beacon system provides a new platform for sensitive and selective detection of various targets including metal ions,small molecules,proteins and other targets. It is potential that this detection method may find a broad spectrum of applications in both environmental and biomedical fields. Acknowledgments
The authors gratefully acknowledge the financial support of the National Natural Science Foundation of China (Nos. 21190044, 21205032,and 91027000),National Natural Science Foundation of Postdoctoral Scientists of China (No. 2013M531779),Hunan Provincial Natural Science Foundation of China (No. 13JJ4032) and the Fundamental Research Funds for the Central Universities of China.
| [1] | J.M. Morgan, D.G. McCormack, M.J. Griffiths, et al., Adenosine as a vasodilator in primary pulmonary hypertension, Circulation 84 (1991) 1145-1149. |
| [2] | F. Costa, I. Biaggioni, Role of nitric oxide in adenosine-induced vasodilation in humans, Hypertension 31 (1998) 1061-1064. |
| [3] | G. Hasko, J. Linden, B. Cronstein, et al., Adenosine receptors: therapeutic aspects for inflammatory and immune diseases, Nat. Rev. Drug Discov. 7 (2008) 759-770. |
| [4] | D.E. Huizenga, J.W. Szostak, A DNA aptamer that binds adenosine and ATP, Biochemistry 34 (1995) 656-665. |
| [5] | W. Xu, Y. Lu, Label-free fluorescent aptamer sensor based on regulation of malachite green fluorescence, Anal. Chem. 82 (2010) 574-578. |
| [6] | L.L. Li, P.H. Ge, P.R. Selvin, Y. Lu, Direct detection of adenosine in undiluted serum using a luminescent aptamer sensor attached to a terbium complex, Anal. Chem. 84 (2012) 7852-7856. |
| [7] | J.B. Liu, Y. Liu, X.H. Yang, et al., Exciton energy transfer-based fluorescent sensing through aptamer-programmed self-assembly of quantum dots, Anal. Chem. 85 (2013) 11121-11128. |
| [8] | A. Ferguson, R.M. Boomer, M. Kurz, et al., A novel strategy for selection of allosteric ribozymes yields riboreporterTM sensors for caffeine and aspartame, Nucleic Acids Res. 32 (2004) 1756-1766. |
| [9] | J. Srinivasan, S.T. Cload, N. Hamaguchi, et al., ADP-specific sensors enable universal assay of protein kinase activity, Chem. Biol. 11 (2004) 499-508. |
| [10] | S. Tyagi, F.R. Kramer, Molecular beacons: probes that fluoresce upon hybridization, Nat. Biotchnol. 14 (1996) 303-308. |
| [11] | K. Wang, Z. Tang, C. Yang, et al., Molecular engineering of DNA: molecular beacons, Angew. Chem. Int. Ed. 48 (2009) 856-870. |
| [12] | J. Huang, X.H. Yang, X.X. He, et al., Design and bioanalytical applications of DNA hairpin-based fluorescent probes, Trends Anal. Chem. 53 (2014) 11-20. |
| [13] | X. Zhang, Z. Wang, H. Xing, Y. Xiang, Y. Lu, Catalytic and molecular beacons for amplified detection of metal ions and organic molecules with high sensitivity, Anal. Chem. 82 (2010) 5005-5011. |
| [14] | L. Lu, X. Zhang, R. Kong, et al., A ligation-triggered DNAzyme cascade for amplified fluorescence detection of biological small molecules with zero-background signal, J. Am. Chem. Soc. 133 (2011) 11686-11691. |
| [15] | X. Zhao, L. Gong, X. Zhang, et al., Versatile DNAzyme-based amplified biosensing platforms for nucleic acid, protein, and enzyme activity detection, Anal. Chem. 85 (2013) 3614-3620. |
| [16] | D. Faulhammer, M. Famulok, The Ca2+ ion as a cofactor for a novel RNA-cleaving deoxyribozyme, Angew. Chem. Int. Ed. 35 (1996) 2837-2841. |
| [17] | R.P. Cruz, J.B. Withers, Y. Li, Dinucleotide junction cleavage versatility of 8-17 deoxyribozyme, Chem. Biol. 11 (2004) 57-67. |
| [18] | J. Achenbach, R. Nutiu, Y.F. Li, Structure-switching allosteric deoxyribozymes, Anal. Chem. Acta 534 (2005) 41-51. |
| [19] | J. Liu, Y. Lu, Adenosine-dependent assembly of aptazyme-functionalized gold nanoparticles and its application as a colorimetric biosensor, Anal. Chem. 76 (2004) 1627-1632. |
| [20] | H. Liu, Y. Xiang, Y. Lu, et al., Aptamer-based origami paper analytical device for electrochemical detection of adenosine, Angew. Chem. Int. Ed. 51 (2012) 6925-6928. |
| [21] | S. Wang, S. Si, Direct fluorescence polarization aptamer-based assay for the determination of adenosine, Anal. Methods 5 (2013) 840-843. |
| [22] | Y. Xiang, A.J. Tong, Y. Lu, Abasic site-containing DNAzyme and aptamer for labelfree fluorescent detection of Pb2+ and adenosine with high sensitivity, selectivity, and tunable dynamic range, J. Am. Chem. Soc. 131 (2009) 15352-15357. |
| [23] | Z.Z. Lü, J.C. Liu, Y. Zhou, et al., Highly sensitive fluorescent detection of small molecules, ions, and proteins using a universal label-free aptasensor, Chem. Commun. 54 (2013) 5465-5467. |






