b UPS, UMR 152 PHARMA-DEV, University of Toulouse III, 118 road of Narbonne, F-31062 Cedex 9 Toulouse, France;
c IRD, UMR 152, F-31062 Cedex 9 Toulouse, France
Heterocycles are a very important class of compounds and an interesting field for research with great opportunities for the synthesis of novel drugs. Pyrimidinone derivatives are widely distributed in nature and exhibit various biological activities such as antimalarials [1, 2, 3],antibacterial [4],antifungal [5],anti-HIV [6], antiviral [7],anticancer [8],and anti-inflammatory agents [9, 10]. In previous reports we demonstrated that aminonitrile naphtopyranique derivatives 1 or 2 are basic substrates for the synthesis of a new series of pyrimidinones [11, 12],and new molecules with a broad range of biological activities,such as triazolopyrimidins [13] and quinolinederivatives [14, 15, 16].Weusedmicrowave assistedsynthesis to control pollution,convert hours into minutes in chemical preparation,enhance yields and stop excessive use of solvents [17].
The present work describes the synthesis of a new series of chloromethylnaphtopyranopyrimidinones 5a-d and 6a-d under mild conditions using microwave irradiation. 2. Experimental
Commercially available,reagent grade chemicals were used as received without additional purification. All reactions were followed by TLC (E. Merck Kieselgel 60 F-254),with detection by UV light at 254 nm. IR spectra were recorded on a Perkin Elmer FT-IR spectrophotometer (4000-400 cm-1) using KBr pellets. The 1H NMR and 13C NMR spectra were recorded on an AC Bruker spectrometer at 300 MHz (1H NMR) and 75 MHz (13C NMR) in deuterated dimethylsulfoxide (DMSO-d6). Chemical shifts (δ) are reported in parts per million (ppm) relative to tetramethylsilane (0 ppm) as internal reference and the following multiplicity abbreviations used: s,singlet; d,doublet; t,triplet; m,multiplet. Mass spectra (MS) were recorded on a spectrometer (Finnigan LCQ Deca XP Max). The ionization method used is electrospray (ESI). All solvents were dried by standard methods. The microwave assisted reactions were carried out in a synthetic microwave apparatus: Monowave 300 with a maximum power of 300 W.
General procedure of method 1: To a solution of 3-amino-1- aryl-1H-benzo[f]chromene-2-carbonitriles 1a-d or 2-amino-4- aryl-4H-benzo[h]chromene-3-carbonitriles 2a-d (0.5 g,1.6 mmol) in DMF (4 mL),chloroacetylchloride (0.36 g,3.2 mmol) was added dropwise at 0 ℃. The reaction mixture was irradiated in a microwave at 40 ℃,for 15-20 min at a power of 150 W. After cooling,100 mL of water were added. A precipitate formed and was filtered,washed with water (3 × 20 mL) and dried in a vacuum drying oven (dessicator cabinet) to give 3a-d or 4a-d. To a mixture of 3a-d or 4a-d (0.1 g,0.26 mmol) and acetone/H2O (1:1,v/v,4 mL) was added K2CO3 (0.053 mmol) and urea hydrogen peroxide (UHP) (0.53 mmol). The reaction mixture was irradiated in a microwave at 70 ℃,for 1.5-2 h at a power of 300 W. After cooling,100 mL of water were added. A precipitate formed and was filtered,washed with water (3 × 20 mL) and dried in a vacuum drying oven (dessicator cabinet) to give compounds 5a-d and 6a-d.
General procedure of method 2: A mixture of 1a-d or 2a-d (1 g, 2.4 mmol) and chloroacetylchloride (6 equiv.) in DMF (4 mL),was irradiated in a microwave at 40 ℃,for 2 h. at a power of 300 W. After cooling,the solid product that formed was filtered,washed with ether,and dried,to give compounds 5a-d or 6a-d in good yields.
Characterization data of the synthesized compounds are listed below.
2-Chloro-N-(2-cyano-1-phenyl-1H-benzo[f]chromen-3-yl)- acetamide (3a): Yellow solid,mp 236-238 ℃; IR (KBr,cm-1): 1670 (C=O),2222 (CN),3340 (NH); 1H NMR(300 MHz,DMSO-d6): δ 4.35 (s,2 H),5.69 (s,1H),7.31-8.01 (m,11H,arom.),11.06 (s,1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 39.7,43.0,85.9,114.8,116.9, 117.3,124.1,125.9,127.8,127.8 (2C),128.0 (2C),129.4 (2C),130.3, 130.5,131.8,143.7,147.3,150.5,165.6; MS (ESI): m/z 373 (M-).
2-Chloro-N-(2-cyano-1-p-methylphenyl-1H-benzo[f]chromen- 3-yl)-acetamide (3b): Yellow solid,mp228-230 ℃; IR (KBr,cm-1): 1723 (C=O),2210 (CN),3324 (NH); 1H NMR (300 MHz,DMSO-d6): δ 2.21 (s,3H),4.35 (s,2H),5.63 (s,1H),7.10-8.00 (m,10H,arom.), 11.02 (s,1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 21.0,39.9,43.0, 86.1,114.9,117.0,117.2,124.2,125.8,127.6,127.9,128.8 (2C), 129.7 (2C),130.3,130.4,131.8,137.1,140.8,147.2,150.3,165.6; MS (ESI): m/z 387 (M-).
2-Chloro-N-(2-cyano-1-p-methoxyphenyl-1H-benzo[f]chromen- 3-yl)-acetamide (3c): Yellow solid,mp 230-232 ℃,IR (KBr, cm-1): 1720 (C=O),2210 (CN),3216 (NH); 1H NMR (300 MHz, DMSO-d6): δ 3.66 (s,3H),4.45 (s,2H),5.68 (s,1H),7.01-8.01 (m, 10H,arom.),11.08 (s,1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 42,3,43.1,55.6,85.7,112.4,114.6,114.6,116.9,117.1,120.2,124.3, 125.8,127.8,129.1 (2C),130.4,130.5,131.8,145.2,147.4,150.88, 159.8,165.7; MS (ESI): m/z 403 (M-).
2-Chloro-N-(2-cyano-1-m-methoxyphenyl-1H-benzo[f]chromen- 3-yl)-acetamide (3d): Yellow solid,mp 226-228 ℃; IR (KBr, cm-1): 1715 (C=O),2220 (CN),3214 (NH); 1H NMR (300 MHz, DMSO-d6): δ 3.70 (s,3H),4.35 (s,2H),5.66 (s,1H),6.79-8.01 (m, 10H,arom.),11.04 (s,1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 39.9,43.0,55.4,85.8,112.5,114.4,114.7,116.9,117.2,120.2,124.2, 125.9,127.8,129.0 (2C),130.4,130.5,131.7,145.1,147.3,150.66, 159.9,165.6; MS (ESI): m/z 403(M-).
2-Chloro-N-(3-cyano-4-phenyl-4H-benzo[h]chromen-2-yl)- acetamide (4a): Yellow solid,mp 220-222 ℃; IR (KBr,cm-1): 1660 (C=O),2200 (CN),3300 (NH); 1H NMR (300 MHz,DMSO-d6): δ 4.45 (s,2H),5.25 (s,1H),7.18-8.16 (m,11H,arom.),11.19 (s,1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 42.4,43.2,83.7,114.6,117.0, 117.5,121.0,123.1,125.6,126.3,127.6 (2C),128.1,128.2,128.4 (2C),129.4 (2C),133.3,143.2,143.7,151.5,165.7; MS (ESI): m/z 373 (M-).
2-Chloro-N-(3-cyano-4-p-methylphenyl-4H-benzo[h]chromen- 2-yl)-acetamide (4b): Yellow solid,mp 214-216 ℃; IR (KBr, cm-1): 1668 (C=O),2210 (CN),3350 (NH); 1H NMR (300 MHz, DMSO-d6): δ 2.27 (s,3H) 4.44 (s,2H),5.19 (s,1H),7.15-8.15 (m, 10H,arom.),11.17 (s,1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 21.0,42.0,43.2,117.0,117.6,121.0,123.1,125.5,126.3,127.5, 128.2,128.3 (2C),129.9 (2C),133.2,137.3,140.9,143.1,151.3, 165.7; MS (ESI): m/z 387 (M-).
2-Chloro-N-(3-cyano-4-p-methoxyphenyl-4H-benzo[h]chromen- 2-yl)-acetamide (4c): Yellow solid,mp 210-212 ℃; IR (KBr, cm-1): 1664 (C=O),2213 (CN),3250 (NH); 1H NMR (300 MHz, DMSO-d6): δ 3.73 (s,3H),4.60 (s,2H),5.69 (s,1H),7.03-8.03 (m, 10H,arom.),11.10 (s,1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 42.1,43.4,55.6,86.7,112.8,114.5,114.7,116.8,117.3,120.2,124.2, 125.9,127.8,129.0 (2C),130.4,130.5,131.8,145.2,147.3,150.6, 159.9,165.7; MS (ESI): m/z 403 (M-).
2-Chloro-N-(3-cyano-4-m-methoxyphenyl-4H-benzo[h]chromen- 2-yl)-acetamide (4d): Yellow solid,mp 212-214 ℃; IR (KBr, cm-1): 1670 (C=O),2200 (CN),3320 (NH); 1H NMR (300 MHz, DMSO-d6): δ 3.74 (s,3H) 4.45 (s,2H),5.22 (s,1H),6.85-8.15 (m, 10H,arom.),11.17 (s,1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 42.3,43.2,55.5,83.7,113.1,114.3,114.5,117.0,117.4,120.5,121.0, 123.1,125.6,127.6,128.0,129.2 (2C),130.6,133.3,145.2,151.6, 160.0,165.8; MS (ESI): m/z 403 (M-).
9-Chloromethyl-12-phenyl-10,12-dihydrobenzo[5,6]chromeno[ 2,3-d]pyrimidin-11-ones (5a): White solid,mp 266-268 ℃; IR (KBr,cm-1): 1663 (C=O),3396 (NH); 1H NMR (300 MHz,DMSOd6): δ 4.46 (s,2H),5.21(s,1H),7.05-8.11 (m,11H,arom.),12.97 (s, 1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 38.3,42.2,101.5,112.5, 119.5,121.5,122.8,124.5,127.4,128.0 (2C),128.4 (2C),129.0 (2C), 129.7,133.9,137.8,143.5,158.6,161.2,166.1; MS (ESI): m/z 373 (M-).
9-Chloromethyl-12-p-methylphenyl-10,12-dihydrobenzo[ 5,6]chromeno[2,3-d]pyrimidin-11-ones (5b): White solid,mp 268-270 ℃; IR (KBr,cm-1): 1683 (C=O),3440 (NH); 1H NMR (300 MHz,DMSO-d6): δ 2.41 (s,3H) 4.38 (s,2H),5.18 (s,1H),7.26- 8.28 (m,10H,arom.),12.78 (s,1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 21.3,41.1,43.2,85.2,105.1,114.5,120.2,121.2,123.6,124.9, 127.1,128.3 (2C),128.9 (2C),129.5 (2C),133.2,137.8,143.8,158.3, 161.7,165.8; MS (ESI): m/z 387 (M-).
9-Chloromethyl-12-p-methoxyphenyl-10,12-dihydrobenzo[ 5,6]chromeno[2,3-d]pyrimidin-11-ones (5c): White solid,mp 266-268 ℃; IR (KBr,cm-1): 1655 (C=O),3390 (NH); 1H NMR (300 MHz,DMSO-d6): δ 3.65 (s,3H) 4.49 (s,2H),5.28 (s,1H),7.19- 8.19 (m,10H,arom.),12.92 (s,1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 38.3,42.6,55.5,85.4,102.8,114.3,119.6,121.1,123.5,124.9, 127.1,127.8 (2C),128.2 (2C),129.4 (2C),133.2,137.7,143.9,158.4, 161.4,166.4; MS (ESI): m/z 403 (M-).
9-Chloromethyl-12-m-methoxyphenyl-10,12-dihydrobenzo[ 5,6]chromeno[2,3-d]pyrimidin-11-ones (5d): White solid,mp 270-272 ℃; IR (KBr,cm-1): 1656 (C=O),3374 (NH); 1H NMR (300 MHz,DMSO-d6): δ 3.66 (s,3H) 4.45 (s,2H),5.24 (s,1H),7.15- 8.15 (m,10H,arom.),12.96 (s,1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 36.8,44.0,55.3,95.7,111.5,114.7,117.3,118.0,120.2,123.8, 125.5,127.5,129.0,129.6,130.1,130.7,131.5,146.6,147.2,148.9, 159.5,165.0,169.2; MS (ESI): m/z 403 (M-).
10-Chloromethyl-7-phenyl-7,9-dihydrobenzo[7,8]chromeno[ 2,3-d]pyrimidin-8-ones (6a): White solid,mp 256-258 ℃; IR (KBr,cm-1): 1665 (C=O),3401 (NH); 1H NMR (300 MHz,DMSO-d6): δ 4.45 (s,2H),5.20 (s,1H),6.91-8.20 (m,11H,arom.),13.01 (s, 1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 37.7,43.4,99.5,113.4, 118.3,119.5,123.4,123.9,127.3,127.8 (2C),128.1(2C),129.5 (2C), 134.4,137.7,143.8,147.3,158.4,163.1,168.1; MS (ESI): m/z 373 (M-).
10-Chloromethyl-7-p-methylphenyl-7,9-dihydrobenzo[7,8]- chromeno[2,3-d]pyrimidin-8-ones (6b): White solid,mp 252- 254 ℃; IR (KBr,cm-1): 1685 (C=O),3442 (NH); 1H NMR (300 MHz, DMSO-d6): δ 2.45 (s,3H) 4.35 (s,2H),5.15 (s,1H),7.25-8.29 (m, 10H,arom.),12.91 (s,1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 21.4,40.3,42.8,102.1,113.9,119.8,121.3,123.4,124.7,127.2, 127.8 (2C),128.4 (2C),129.5 (2C),133.5,137.9,143.8,147.3,158.5, 165.1,167.4; MS (ESI): m/z 387 (M-).
10-Chloromethyl-7-p-methoxyphenyl-7,9-dihydrobenzo[7,8]- chromeno[2,3-d]pyrimidin-8-ones (6c): White solid,mp 250- 252 ℃; IR (KBr,cm-1): 1682 (C=O),3387 (NH); 1H NMR (300 MHz, DMSO-d6): δ 3.66 (s,3H) 4.45 (s,2H),5.24 (s,1H),7.15-8.15 (m, 10H,arom.),12.96 (s,1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 38.2,42.6,55.4,102.8,114.2,119.6,121.1,123.5,124.9,127.1, 128.1 (2C),128.9 (2C),129.4 (2C),133.1,137.7,143.9,147.6,158.4, 161.4,167.2; MS (ESI): m/z 403 (M-).
10-Chloromethyl-7-m-methoxyphenyl-7,9-dihydrobenzo[7,8]chromeno[2,3-d]pyrimidin-8-ones (6d): White solid,mp 250-252 ℃; IR (KBr,cm-1): 1683 (C=O),3350 (NH); 1H NMR (300 MHz,DMSO-d6): δ 3.62 (s,3H) 4.44 (s,2H),5.27 (s,1H),7.11- 8.11 (m,10H,arom.),12.80 (s,1H,NH); 13C NMR (75 MHz,DMSO-d6): δ 38.5,42.7,55.5,101.9,113.3,119.6,121.3,123.6,124.8, 127.1,128.1 (2C),128.8 (2C),129.5 (2C),133.2,137.6,143.8,147.6, 158.4,161.5,167.6; MS (ESI): m/z 403 (M-). 3. Results and discussion
The compounds,9-chloromethyl-12-aryl-10,12-dihydrobenzo[5,6]chromeno[2,3-d]pyrimidin-11-ones 5a-d and 10-chloromethyl- 7-aryl-7,9-dihydrobenzo[7,8]chromeno[2,3-d]pyrimidin- 8-ones 6a-d,were synthesized by two different methods.
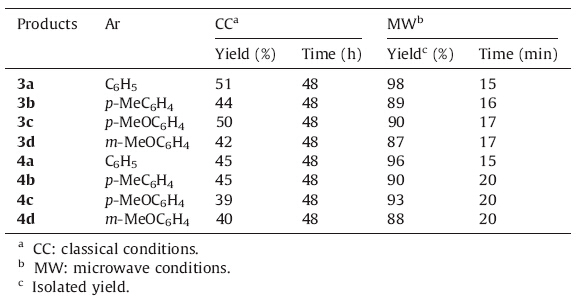
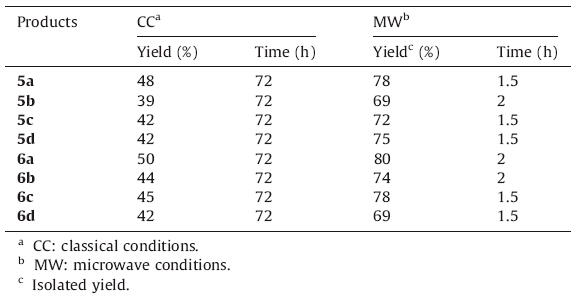
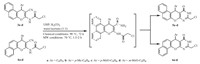
In the first method,the synthesis of compounds 5 and 6 involves two steps. Starting materials 3-amino-1-aryl-1H-benzo[f]chromene- 2-carbonitriles 1a-d or 2-amino-4-aryl-4H-benzo[h]chromene-3-carbonitriles 2a-d reacted with excess of chloroacetylchloride (2 equiv.) to afford 2-chloro-N-(2-cyano-1-aryl-1H-benzo[f]chromen-3-yl)- acetamide 3a-d or 2-chloro-N-(3-cyano-4-aryl-4H-benzo[h]chromen- 2-yl)-acetamide 4a-d,followed by Radziszewski’s reaction using urea hydrogen peroxide (UHP) as a mild,safe and non-hazardous oxidizing agent,leading to compounds 5 and 6.We proceeded initially by conducting the first step in both classical and microwave-assisted conditions,to compare these twomethods (Scheme 1).Under classical conditions,compounds 3 and 4 are obtained in 39%-51% yields after 48 h reaction time,while with microwave irradiation the same products are isolated in 87%-98% yields after only 15-20 min reaction time (Table 1). We then compared classical conditions versus microwave-assisted procedures for the second step of the first method. Inclassical conditions (72 hat 84 ℃),the derivatives 5a-d and 6a-d are obtained in 39%-50% yields,and undermicrowave irradiation (2 h,reaction time at 70 ℃),thesame products are prepared in69-80% yields (Table 2). The mechanism of transformation of substrates 3 and 4 to products 5 and 6 proceed via oxidation of the nitrile function to amide followed by cyclization (Scheme 2).

|
Download:
|
| Scheme 1.General synthetic route of the compounds 3a-d and 4a-d. | |
|
Download:
|
| Scheme 2.General synthetic route of the target compounds 5a-d and 6a-d. | |
| Table 1 Microwave-assisted synthesis of compounds 3a-d and 4a-d. |
| Table 2 Microwave-assisted synthesis of compounds 5a-d and 6a-d with method 1. |
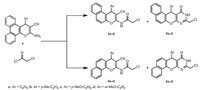
In the second method,a one step reaction allows the synthesis of pyrimidinones derivatives 5a-d and 6a-d (Scheme 3).
|
Download:
|
| Scheme 3.General synthetic route of the target compounds 5a-d and 6a-d in one step. | |
Condensation of compounds 1a-d or 2a-d with a large excess (6 equiv.) of chloroacetylchloride provides the final product 5 or 6. In this study,we investigate the reaction under classical conditions by varying the temperature and reaction time. After several assays, the optimum result is obtained when the reaction is carried out in DMF for seven days,at room temperature,but unfortunately,the yields (32%-35%) obtained were much lower than those previously observed. Under microwave irradiation,the best results are obtained in DMF at 40 ℃ during 120 min of reaction (44%-56%) (Table 3). However,it was very difficult to determine conditions providing 5 or 6 without traces of intermediates 3 and 4,even by increasing reaction time and temperature.
| Table 3 General synthetic route of the target compounds 5a-d and 6a-d in one step. |
In the present study,we have investigated the preparation of new naphtopyranopyrimidinones 5a-d and 6a-d using microwave irradiation. This method is easily and rapidly performed and affords these compounds in very good yield. A second method allows us to readily synthesize,in one step,the same compounds from aminonitrile naphtopyranque and chloroacetylchloride. In conclusion,the microwave assisted methods offer significant advantages for the rapid synthesis in high yield and purity of all compounds presented in this study. The required reaction times and volumes of solvents are much lower.
AcknowledgmentsThe authors acknowledge the Ministry of Higher Education, Scientific Research and Technology of Tunisia and the University of Toulouse III for financial support.
| [1] | C. Santelli-Rouvier, B. Pradines, M. Berthelot, et al., Arylsulfonyl acridinyl derivatives acting on plasmodium falciparum, Eur. J. Med. Chem. 39 (2004) 735-744. |
| [2] | A.K. Bhattacharjee, M.G. Hartell, D.A. Nichols, et al., Structure-activity relationship study of antimalarial indolo [2,1-b]quinazoline-6,12-diones (tryptanthrins). Three dimensional pharmacophore modeling and identification of new antimalarial candidates, Eur. J. Med. Chem. 39 (2004) 59-67. |
| [3] | P. Verhaeghe, N. Azas, S. Hutter, et al., Synthesis and antiplasmodial activity of new 4-aryl-2-trichloromethylquinazolines, Bioorg. Med. Chem. 17 (2008) 396-401. |
| [4] | A.S. Hossan, H.M. Abu-Melha, M.A. Al-Omar, et al., Synthesis and antimicrobial activity of some new pyrimidinone and oxazinone derivatives fused with thiophene rings using 2-chloro-6-ethoxy-4-acetylpyridine as starting material, Molecules 17 (2012) 13642-13655. |
| [5] | M. Ashok, B.S. Holla, N.S. Kumari, Convenient one pot synthesis of some novel derivatives of thiazolo[2,3-b]dihydropyrimidinone possessing 4-methylthiophenyl moiety and evaluation of their antibacterial and antifungal activities, Eur. J. Med. Chem. 42 (2007) 380-385. |
| [6] | F. Hamy, V. Brondani, A. Flörsheimer, et al., A new class of HIV-1 Tat antagonist acting through Tat TAR Inhibition, Biochemistry 37 (1998) 5086-5095. |
| [7] | A.S. Galabov, E. Velichkova, A. Karparov, et al., Antiviral activity of tetrahydro-2(1H)-pyrimidinones and related compounds, Arzneimittelforschung 34 (1984) 9-14. |
| [8] | S. Karkola, A. Lilienkampf, K. Wähälä, A 3D QSAR model of 17b-HSD1 inhibitors based on a thieno[2,3-d]pyrimidin-4(3H)-one core applying molecular dynamics simulations and ligand-protein docking, Chem. Med. Chem. 3 (2008) 461-472. |
| [9] | E.S. Lee, J.G. Park, Y. Jahng, A facile synthesis of simple alkaloids-synthesis of 2,3-polymethylene-4(3H)-quinazolinones and related alkaloids, Tetrahedron Lett. 44 (2003) 1883-1886. |
| [10] | G. Romeo, F. Russo, A. Caruso, et al., Synthesis of new thieno[2,3-d]pyrimidine-2,4(1H,3H)-diones with analgesic and anti-inflammatory activities, Arzneimittelforschung 48 (1998) 167-172. |
| [11] | M. Messaâd, F. Chabchoub, M. Salem, Action of primary amines and hydroxylamine on ethoxymethyleneaminonaphtopyranes: synthesis of new naphtopyrano[2,3-d] pyrimidines derivatives, Heterocycl. Commun. 9 (2005) 401-404. |
| [12] | K. Mkaouar, F. Chabchoub, A. Samadi, et al., Convenient synthesis of 11-aryl-1,12-dihydro11H-naphthopyrano[2,3-d]pyrimidin-12-ones, Synth. Commun. 40 (2010) 3405-3414. |
| [13] | F. Chabchoub, M. Messaâd, H.B. Mansour, L. Chekir-Ghedira, M. Salem, et al., Synthesis and antigenotoxic activity of some naphtho[2,1-b]pyrano[3,2-e][1,2,4]triazolo[1,5-c]pyrimidine derivatives, Eur. J. Med. Chem. 42 (2007) 715-718. |
| [14] | E. Maalej, F. Chabchoub, A. Samadi, et al., Synthesis, biological assessment and molecular modeling of 14-aryl-10,11,12,14-tetrahydro-9H-benzo[5,6]chromeno[2,3-b]quinolin13-amines, Bioorg. Med. Chem. Lett. 21 (2011) 2384-2388. |
| [15] | E. Maalej, F. Chabchoub, M.J. Oset-Gasque, et al., Synthesis, biological assessment, and molecular modeling of racemic 7-aryl-9,10,11,12-tetrahydro-7H-benzo[7,8]-chromeno[2,3-b]quinolin-8-amines as potential drugs for the treatment of Alzheimer's disease, Eur. J. Med. Chem. 54 (2012) 750-763. |
| [16] | M. Esquivias-Pérez, E. Maalej, A. Romero, et al., Nontoxic and neuroprotective bnaphthotacrines for Alzheimer's disease, Chem. Res. Toxicol. 26 (2013) 986-992. |
| [17] | A. Diaz-Ortiz, A. De La Hoz, A. Moreno, Selectivity in organic synthesis under microwave irradiation, Curr. Org. Chem. 8 (2004) 903-918. |