Quinoxaline and its derivatives are an important class of heterocycles which have a variety of applications in many fields, such as antibacterials [1, 2, 3],electroluminescent materials [4], organic semiconductors [5] and useful intermediates in organic synthesis [6, 7, 8]. In light of this significance,a number of synthetic strategies have been developed for the preparation of substituted quinoxalines from a-hydroxyketones and diamines,in which diverse catalysts such as CuCl2 [9],Pd(OAc)2,RuCl2(PPh3)2 [10], and FeCl3 [11] have been reported. Even though these existing catalysts have advantages,they face the difficulty of recycling. Recently,Lingaiah et al. [12] reported a heterogeneous process utilizing the iron exchanged molybdophosphoric acid (FePMA) as a catalyst. The catalyst is efficient and recoverable,but the reaction requires an oxygen atmosphere,and the catalytic efficiency decreases significantly upon reuse. Therefore,it has become a very important task to obtain a stable and recyclable catalyst for the highly efficient synthesis of quinoxaline.
Rare earth catalysts showed several advantages in some important organic reactions [13, 14, 15],such as heterocyclic synthesis, in our previous study [16]. Y zeolite has large specific area,good thermal stability,and solid acidity,which make it widely used as a substrate [17]. In this study,we report the ion-exchange fabrication of Yb immobilized NaY zeolite catalyst (Yb/NaY) by a hydrothermal method [18] and an application of this catalyst for the synthesis of quinoxaline. The catalyst gave the desired products in good to excellent yields (up to 94%) and could be reused several times without any loss of its catalytic activity. This finding provides an example of NaY-based zeolite as an efficient catalyst for widespread applications of quinoxaline synthesis. 2. Experimental
General procedure for catalyst preparation: Powdered NaY (8.3 g) was dispersed in distilled water (160 mL) and then mixed with desiccative YbCl3 (8.2 g) under stirring for 0.5 h. After stirring for 0.5 h,the mixture was transferred into a 200 mL PTFE autoclave,sealed,and heated in a hot air oven at 180 ℃ for 1 h. The solid product was recovered by filtration and washed with an excess of distilled water,then dried at 100 ℃ in an oven. The dried sample was calcined from room temperature to 550 ℃ at a heating rate of 10 ℃/min and held at 550 ℃ for 5 h. Finally,the sample was cooled to ambient temperature.
Typical procedure for the condensation synthesis of compound 3a: Benzoin 1a (1.0 equiv.),1,2-diamine 2a (2.0 equiv.),oxidant (0.35 mol%),and Yb/NaY catalyst (Yb3+ 0.05 equiv.) were reacted in solvent (5 mL) in a 25 mL round bottom flask. The mixture was stirred for 4 h at 80 ℃. After completion of the reaction,the mixture was centrifuged and washed with ethyl acetate (3 times × 10 mL). The solid catalyst was recovered and dried at 80 ℃ for 2 h. The combined organic solution was evaporated under reduced pressure and purified by column chromatography on silica gel,eluting with petroleum ether/ethyl acetate (20:1,v/v),to get 2,3-diphenyl-quinoxaline 3a. Other products were synthesized through typical procedures. All 1H NMR results are summarized in Supporting information. The configuration of compounds 3a-3n was assigned by comparing 1H NMR data with known compounds [19, 20, 21, 22, 23]. 3. Results and discussion
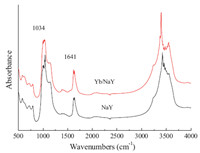
The FTIR spectra of NaY and Yb/NaY are presented in Fig. 1. Characteristic peaks of Yb/NaY are basically the same with parent NaY,except a slight shift due to the introduction of Yb3+. The peaks around 1034 cm-1 and 1641 cm-1 are assigned to Si-O-Si asymmetric stretching vibration and H-O-H symmetric stretching vibration,respectively. Peaks between 600 cm-1 and 800 cm-1 correspond to the internal and external symmetric stretching vibration of TO4 tetrahedron (T = Si or Al) [24]. This result shows that the zeolite skeleton and crystal structure of NaY remain intact, as supported by the XRD patterns of the same samples (Fig. 2).
|
Download:
|
| Fig. 1.IR spectra of parent NaY and Yb/NaY. | |

|
Download:
|
| Fig. 2.XRD patterns of parent NaY and Yb/NaY. | |
From XRD analysis,all the peaks of the Yb/NaY can be indexed to the diffraction peak of NaY at a slightly higher 2θ region,and these narrow peaks suggest that the Yb/NaY particles are highly crystalline according to the Scherrer equation. Besides,no significantly different peak is observed between the two diffraction patterns,which indicate that the Yb3+ was finely dispersed throughout the NaY zeolite.
Fig. 3 shows the SEM images of the parent NaY and ytterbium modified NaY. These images show similar morphology and wellordered porous structure,as well as favorable dispersity,suggesting that the loading of ytterbium has a negligible effect on the main structure of the NaY zeolite. Hence,even when the Yb doped concentration is up to 10.6 wt%,detected by ICP-AES,the supported zeolite still has a structure similar to NaY. This implies Yb/NaY zeolite effectively doped high concentrations of rare earth metal.

|
Download:
|
| Fig. 3.SEM images of parent NaY (a) and Yb/NaY (b). | |
According to the BET results,the specific surface areas of parent NaY and Yb/NaY are 404 m2/g and 487 m2/g,respectively,which means modification increased the specific surface area of NaY. Based on previous reports [25, 26],Yb3+ could migrate to the sodalite cage of NaY zeolite through hydrothermal method,and this would improve its surface area,as well as generate charge effect and change the microenvironment of reaction sites. These features of Yb/NaY may affect its catalytic performance.
The NH3-TPD results are listed in Table 1. Compared with parent NaY zeolite,the acid amount of modified NaY zeolite was significantly higher,especially in the middle-strong acid amount, which means the introduction of Yb3+ has a significant effect on the surface acid of Y zeolite. The change in acidity indicates that the synthesized catalyst may have better catalytic activity in addition to being recyclable.
| Table 1 NH3-TPD results of NaY and Yb/NaY. |
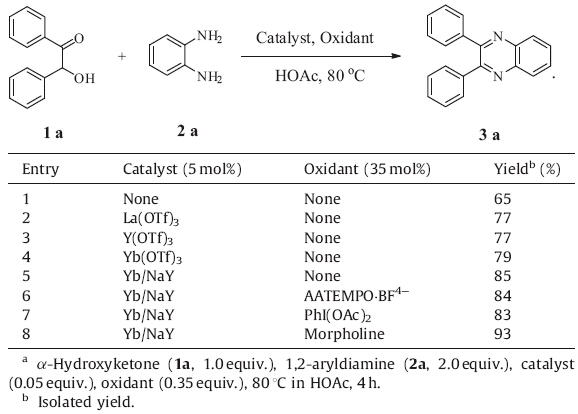
To test its catalytic efficiency,the Yb/NaY was further applied to condensation reactions for the synthesis of quinoxalines. The results are summarized in Table 2. Synthesis of 2,3-diphenylquinoxaline 3a was chosen as a model reaction to optimize the reaction conditions. It turned out that in the absence of the catalyst,the product was obtained in only 65% yield (Table 2,entry 1). With the addition of Ln(OTf)3,the yields improved to 79% (Table 2,entries 2-4). While with the catalysis of Yb/NaY,the yield was up to 85% (Table 2,entry 5). To further improve the yield, various oxidants were added into the model reaction (Table 2, entries 6-8). The results revealed that morpholine is the most efficient oxidant,and with it the highest yield (93%) was obtained.
| Table 2 Optimization of model reaction and comparison of catalysts.a |
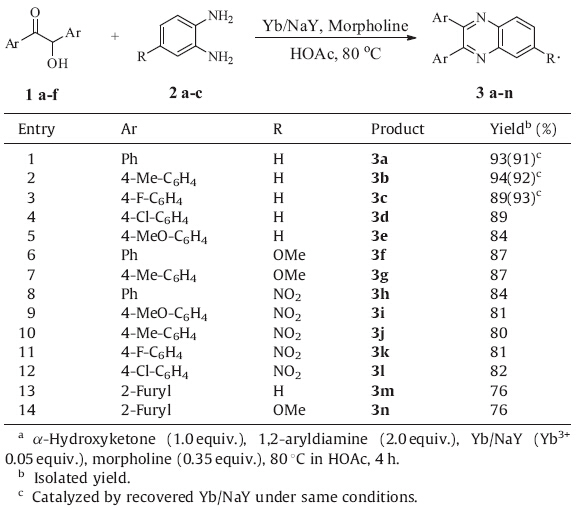
Encouraged by the results,the synthetic protocol and scope were further explored by employing a variety of 1,2-aryldiamines (1 equiv.) and α-hydroxyketones (2 equiv.) (Table 3). In all cases, the reaction gave the corresponding products in good yields. Importantly,the solid catalyst in several reactions can be recycled by simple centrifugation with a recovery rate of more than 95%. When applying the recycled Yb/NaY in the same reactions (Table 3, entries 1-3),the yields of the corresponding products remain unchanged or even improved. It is inferred that the catalysts was activated in the first run.
| Table 3 Synthesis of quinoxaline derivatives catalyzed by Yb/NaY under the optimal condition.a |
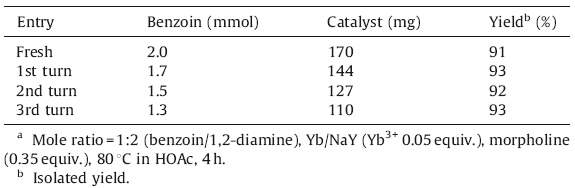
Reusability of the catalyst was studied through the model reaction under optimized conditions. The reaction proceeded smoothly with a yield of 93%-91% (Table 4). This result indicates that the Yb/NaY catalyst has a good comparability with traditional catalysts in terms of yields and reusability.
| Table 4 Reuse of the catalyst for synthesis of 3a.a |
In this study,the Yb/NaY catalyst for the condensation synthesis of quinoxaline was obtained by a simple hydrothermal method. Both structural and chemical features made it efficient and recyclable. The catalyzed reactions gave the desired products in good to excellent yields,and the catalyst could be reused several times without any loss of its high catalytic activity. This finding highlights the potential of the Yb/NaY zeolite as an efficient catalyst for widespread applications.
AcknowledgmentWe gratefully acknowledge the National Natural Science Foundation of China (No. 20802052) for financial support.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.03.003.
| [1] | A. Dell, D.H. William, H.R. Morris, et al., Structure revision of the antibiotic echinomycin, J. Am. Chem. Soc. 97 (1975) 2497-2502. |
| [2] | W. He, M.R. Meyers, B. Hanney, et al., Potent quinoxaline-based inhibitors of PDGF receptor tyrosine kinase activity. Part 2: The synthesis and biological activities of RPR127963 an orally bioavailable inhibitor, Bioorg. Med. Chem. Lett. 13 (2003) 3097-3100. |
| [3] | Y.B. Kim, Y.H. Kim, J.Y. Park, S.K. Kim, Synthesis and biological activity of new quinoxaline antibiotics of echinomycin analogues, Bioorg. Med. Chem. Lett. 14 (2004) 541-544. |
| [4] | K.R.J. Thomas, M. Velusamy, J.T. Lin, C.H. Chuen, Y.T. Tao, Chromophore-labeled quinoxaline derivatives as efficient electroluminescent materials, Chem. Mater. 17 (2005) 1860-1866. |
| [5] | S. Dailey, W.J. Feast, R.J. Peace, et al., Synthesis and device characterisation of sidechain polymer electron transport materials for organic semiconductor applications, J. Mater. Chem. 11 (2001) 2238-2243. |
| [6] | K.B. Woody, B.J. Leever, M.F. Durstock, D.M. Collard, Synthesis and characterization of fully conjugated donor-acceptor-donor triblock copolymers, Macromolecules 44 (2011) 4690-4698. |
| [7] | M. Wang, Y. Li, H. Tong, et al., Hexaazatriphenylene derivatives with tunable lowest unoccupied molecular orbital levels, Org. Lett. 13 (2011) 4378-4381. |
| [8] | Y. Shirai, A.J. Osgood, Y.M. Zhao, et al., Surface-rolling molecules, J. Am. Chem. Soc. 128 (2006) 4854-4864. |
| [9] | C.S. Cho, S.G. Oh, Copper-catalyzed oxidative cyclization of α-hydroxyketones with o-phenylenediamines leading to quinoxalines, J. Mol. Catal. A: Chem. 276 (2007) 205-210. |
| [10] | R.S. Robinson, R.J.K. Taylor, Quinoxaline synthesis from α-hydroxy ketones via a tandem oxidation process using catalyzed aerobic oxidation, Synlett 6 (2005) 1003-1005. |
| [11] | W.B. Song, P. Liu, M. Lei, et al., FeCl3 and morpholine as efficient cocatalysts for the one-step synthesis of quinoxalines from α-hydroxyketones, and 1,2-diamines, Syn. Commun. 42 (2012) 236-245. |
| [12] | K.T.V. Rao, P.S.S. Prasad, N. Lingaiah, Iron exchanged molybdophosphoric acid as an efficient heterogeneous catalyst for the synthesis of quinoxalines, J. Mol. Catal. A: Chem. 312 (2009) 65-69. |
| [13] | S. Kobayashi, M. Sugiura, H. Kitagawa, W.W.L. Lam, Rare-earth metal triflates in organic synthesis, Chem. Rev. 102 (2002) 2227-2302. |
| [14] | L.M. Wang, J.J. Xia, F. Qin, T.C. Qian, J. Sun, Yb(OTf)3-catalyzed one-pot synthesis of quinazolin-4(3H)-ones from anthranilic acid, amines and ortho esters (or formic acid) in solvent-free conditions, Synthesis 8 (2003) 1241-1247. |
| [15] | X.M. Ma, B.D. Li, M. Lu, C.X. Lv, Rare earth metal triflates catalyzed electrophilic nitration using N2O5, Chin. Chem. Lett. 23 (2012) 73-76. |
| [16] | L.Y. Fan, W. Chen, C.T. Qian, YbCl3-catalyzed one-pot synthesis of dihydropyrazines, piperazines, and pyrazines, Tetrahedron Lett. 54 (2013) 231-234. |
| [17] | A. Khorshidi, Indole cyanation via C-H bond activation under catalysis of Ru(Ⅲ)-exchanged NaY zeolite (RuY) as a recyclable catalyst, Chin. Chem. Lett. 23 (2012) 903-906. |
| [18] | J. Scherrer, J.L. Bass, F.D. Hunter, Structural characterization of hydrothermally treated lanthanum Y zeolites. 1. Framework vibrational spectra and crystal structure, J. Phys. Chem. 79 (1975) 1194-1199. |
| [19] | W.X. Guo, H.L. Jin, J.X. Chen, et al., An efficient catalyst-free protocol for the synthesis of quinoxaline derivatives under ultrasound irradiation, J. Braz. Chem. Soc. 20 (2009) 1674-1679. |
| [20] | M.M. Heravi, M. Hosseini, H.A. Oskooie, B. Baghernejad, Fe/Al-MCM-41: an efficient and reusable catalyst for the synthesis of quinoxaline derivatives, J. Korean Chem. Soc. 55 (2011) 235-239. |
| [21] | J.F. Zhou, G.X. Gong, S.J. Zhi, X.L. Duan, Microwave-assisted catalyst-free and solvent-free method for the synthesis of quinoxalines, Synth. Commun. 39 (2009) 3743-3754. |
| [22] | B. Karami, S. Khodabakhshi, M. Nikrooz, Synthesis of aza-polycyclic compounds: novel phenazines and quinoxalines using molybdate sulfuric acid (MSA), Polycycl. Aromat. Comp. 31 (2011) 97-109. |
| [23] | S. Sithambaram, Y.S. Ding, W.N. Li, et al., Manganese octahedral molecular sieves catalyzed tandem process for synthesis of quinoxalines, Green Chem. 10 (2008) 1029-1032. |
| [24] | E.M. Flanigen, H. Khatami, H.A. Seymenski, Infrared structural studies of zeolite frameworks, in: E.M. Flanigen, L.B. Sand (Eds.), Molecular Sieve Zeolites-I, American Chemical Society, Washington, DC, 1974, pp. 201-229. |
| [25] | D.H. Yu, P. Sun, Z.C. Tang, Z.X. Li, H. Huang, Modification of NaY La3+ for the dehydration of lactic acid: the effect of preparation protocol on catalyst microstructure and catalytic performance, Can. J. Chem. Eng. 89 (2011) 484-490. |
| [26] | H.J. Wang, D.H. Yu, P. Sun, et al., Rare earth metal modified NaY: structure and catalytic performance for lactic acid dehydration to acrylic acid, Catal. Commun. 9 (2008) 1799-1803. |