b School of Marine Science and Engineering, Tianjin University of Science and Technology, Tianjin 300457, China
Gelatin is derived from the waste,or by-products,of manufacturing processes comprising tannery,pharmaceutical and food segments. It can be taken as a renewable and biodegradable material [1, 2]. However,its applications are limited in many areas due to its poor antibacterial activity,brittleness and poor water solubility. Chemical grafting or cross-linking of gelatin is an effective way to introduce stable covalent bonds between protein segments to improve the chemical and physical properties of natural polymers [3, 4, 5]. The epoxy compounds are commonly used in modified research.
Vargas et al. [1] studied the cross-linking of gelatin by ethylene glycol diglycidyl ether (EGDE). The best cross-linking reaction between gelatin and EGDE was achieved at pH9 and an EGDE concentration of 10 wt%. The cross-linking structures were confirmed by FT-IR and 13C NMR techniques. Xu et al. [6],studied the microstructural transformations of PGG polymers which were based on α-[3-(2,3-epoxy-propoxy)propyl]-w-butylpolydimethyl siloxanes (PDMS-E) and gelatin induced by sodium dodecyl sulfate (SDS) and sodium dodecyl benzene sulfonate (SDBS). The results indicated that the microstructural transformation of PGG was determined by the compatibility of the two polymers in anionic surfactant aqueous solution and the chemical nature of their monomers.
In the present work,chemical modification of gelatin by epoxypropyl dodecyl dimethyl ammonium chloride (EPDDMAC) and diethyl-2,3-epoxypropyl-[3,methyldimethoxyl] silpropyl ammonium chloride (DEEPSAC) at varying the gelatin concentrations in water is studied. The grafting density of epoxy quaternary ammonium salts grafted gelatin polymers is investigated using the Van Slyke method by measuring the conversion rate of free NH2 groups of gelatin [3]. The structure of modified gelatin is characterized by FT-IR,13C NMR and elemental analysis. The glass-transition temperatures Tg,contact angles and antibacterial properties are reported. 2. Experimental
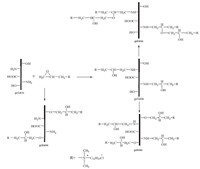
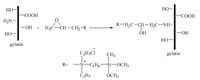
The grafting route of EPDDMAC-GE was depicted in Scheme 1. Gelatin (type A obtained from pigskin,having an approximate molecular weight of 50,000 and isoelectric point at pH8 determined by fluorescence measurements) was dissolved in distilled water (8%,ws/wg),and after 5 h,the gelatin solution was heated to 50 ℃ to ensure complete dissolution. The pH of the solution was adjusted to 10.0 by NaOH (2.0 mol/L) solution [7]. The EPDDMAC (laboratory-made compounds,mp. -0.28 ℃,purity 98.6%) with predetermined epoxy groups/amino groups (1:1,mol:mol) was then slowly added to the gelatin solution at 50 ℃ with stirring. After reacting for 5 h,the solution was cooled to 5 ℃ and the content of free -NH2 groupswas determinedby the improved Van Slyke method at 40 ℃ [8]. The grafting density of -NH2 groups reached 40.03%. The grafting route of DEEPSAC-GE was depicted in Scheme 2. Gelatin was dissolved in distilledwater (6%,ws/wg),and after 5 h,the gelatin solution was heated to 55 ℃ to ensure complete dissolution. The pH of the solution was adjusted to 11.0 by NaOH (2.0 mol/L) solution. The DEEPSAC (laboratory-made compounds,purity 98.9%) with predetermined epoxy groups/amino groups (1:1,mol:mol) was then slowly added to the gelatin solution at 55 ℃with stirring. The conversion of -NH2 groups reached 42.15%. All samples were freeze dried at -50 ℃ for 24 h and extracted by acetone for 72 h [6]. The chemical structures of the gelatin and its derivatives were determined by FT-IR spectra (Nicolet NEXUS 470 FT-IR spectrometer),13C NMR spectra (Bruker Advance 400 spectrometer) and elemental analysis (Vario EL Ⅲ,Elementar Analysen System GmbH, Germany).
|
Download:
|
| Scheme 1.The synthetic routes of EPDDMAC-GE polymers. | |
|
Download:
|
| Scheme 2.The synthetic route of DEEPSAC-GE polymers. | |
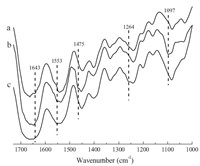
Milch [9] investigated any indications of gross changes in the infrared spectra of the gelatin system with gel melting. In this study,the chemical structures of the gelatin and its derivatives were also determined by the infrared spectra. Fig. 1 is the infrared spectra of three polymers. Comparing the spectrum of EPDDMACGE (b) with the spectrum of gelatin (a),the absorption band at 1643 cm-1 (belonging to the N-H deformation of primary amine groups on gelatin chains) nearly disappeared,implying that the epoxide group of EPDDMAC has been coupled with the -NH2 groups of gelatin. Concomitantly,there was a new peak observed at 1553 cm-1 (belonging to the N+-C group) and the absorption peak at 1475 cm-1 (due to the C-H functional groups of the methylene substituent of quaternary ammonium groups) appearing in the spectrum of EPDDMAC-GE. It can be concluded that quaternary ammonium substituents were successfully introduced into the gelatin backbone. The peak at 1097 cm-1 (assigned to symmetric vibration of C-O-C stretching shifted in the EPDDMAC-GE) appeared,demonstrating that the -OH groups of gelatin participated in the formation of ether bond in gelatin backbone. Comparing the spectrum of DEEPSAC-GE (c) with the spectrum of gelatin (a),the peak at 1643 cm-1 (belonging to the N-H deformation of primary amino groups on gelatin chains) disappeared, implying that the epoxide groups of DEEPSAC have been coupled with the -NH2 groups of gelatin. The new peaks at 1553 cm-1 and 1264 cm-1 ascribed to N+-C group and Si-C group, respectively,of the quaternary ammonium salt appeared,which demonstrated that quaternary ammonium substituent was introduced in the gelatin backbone.
|
Download:
|
| Fig. 1.The FT-IR spectra of (a) initial gelatin,(b) EPDDMAC-GE and (c) DEEPSAC-GE. | |
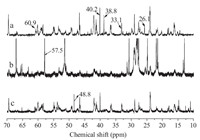
As shown in Fig. 2,some of the modifications taking place in the gelatin molecule could be observed by 13C NMR spectroscopy. Comparing the NMR spectrum of EPDDMAC-GE (b) with the spectrum of gelatin (a),the peaks of arginine (δ-CH2,38.8 ppm) and lysine (ε-CH2,40.2 ppm) of EPDDMAC-GE disappeared. The results indicated that the -NH2 groups had completely reacted with the epoxide groups of EPDDMAC. The peaks of hydroxyproline (b-CH2, 33.1 ppm) and proline (α-CH2,60.9 ppm) of EPDDMAC-GE also disappeared,indicating that the -OH groups of gelatin were involved in the grafting process and the N+-C (57.5 ppm) of EPDDMAC-GE appeared. It can be concluded that EPDDMAC was successfully introduced into the gelatin backbone. Comparing the spectrum of DEEPSAC-GE (c) with the spectrum of gelatin (a), peaks at arginine (δ-CH2,38.8 ppm) and lysine (δ-CH2,26.1 ppm) of DEEPSAC-GE disappeared,implying that the epoxide group of DEEPSAC had been completely coupled with the -NH2 groups of gelatin. In the branched system,α-N+ would shift (△δ = 0.5-9 ppm) [10] upon protonation of the nitrogen atom to produce the shielding effect,so N+-C of DEEPSAC-GE appeared at 48.8 ppm. It can be concluded that DEEPSAC was successfully introduced into the gelatin backbone.
|
Download:
|
| Fig. 2.The 13C NMR spectra of (a) initial gelatin,(b) EPDDMAC-GE and (c) DEEPSACGE. | |
Table 1 shows the elemental analysis of the gelatin and its derivatives. The calculated values were obtained from the conversion of -NH2 groups and the determined values of initial gelatin. Based on the FT-IR,13C NMR and elemental analysis,it is concluded that the epoxy group of EPDDMAC mainly reacts with the free -NH2 groups in the gelatin chains (Ⅰ,Scheme 1) and a small quantity of -OH groups is also involved in the reaction (Ⅱ,Ⅲ,Ⅳ, Scheme 1). While the DEEPSAC only reacts with the free -NH2 groups (Ⅰ,Scheme 2) and does not react with -OH groups. The reason is that EPDDMAC,which contains an alkyl chain,is more active than DEEPSAC which contains Si-C bonds. In an alkaline environment,EPDDMAC reacts relatively easily with the activated -NH2 groups [11].
| Table 1 The elemental analysis of EPDDMAC-GE and DEEPSAC-GE. |
The glass-transition temperatures of the EPDDMAC-GE and DEEPSAC-GE were measured by conventional DSC (SDTQ600,TA Instrument,USA). After grafting onto gelatin,interchaining hydrogen bonds between C=O and N-H groups are reduced due to the decrease of free -NH2,which can lead to the decrease of Tg [12]. Fig. 3 shows that Tg of initial gelatin (a) is 229.39 ℃. It is higher than that of the modified gelatin at 2% (ws/wg) DEEPSAC-GE (b,220.41 ℃); 6% (ws/wg) DEEPSAC-GE (c,215.37 ℃); 2% (ws/wg) EPDDMAC-GE (d,204.23 ℃); 8% (ws/wg) EPDDMAC-GE (e, 186.98 ℃). The glass-transition temperatures (Tg) of the polymers decreased gradually with the increase of the grafting density, which is simply affected by the branching effect on the gelatin matrix.

|
Download:
|
| Fig. 3.The DSC of (a) initial gelatin; (b) 2% (ws/wg) DEEPSAC-GE; (c) 6% (ws/wg) DEEPSAC-GE; (d) 2% (ws/wg) EPDDMAC-GE; (e) 8% (ws/wg) EPDDMAC-GE. | |
The films were obtained from the aqueous solution of gelatin and its derivatives dropped on the glass and dried in a vacuum oven at 25 ℃ for 48 h. The thickness of the films is about 3-5 mm. The water contact angles (CAs) were measured by the Sessile drop method using a DSA100 contact angle measuring system from Kru¨ ss. Fig. 4 is the camera images of 4 μL water droplets on the films. Their corresponding contact angles (CAs) are listed in Fig. 4. The CAs of EPDDMAC-GE films (as shown in d-f) are much smaller than the CAs of the initial gelatin film (as shown in a) for the hydrophilic property of gelatin increases with the number of quaternary ammonium groups introduced. Whereas,the CAs of DEEPSAC-GE films (as shown in b and c) are higher than that of initial gelatin film and increased gradually with the increase of the grafting density. The quaternary ammonium group containing organosilicon group has low surface free energy,it can gather to the film surface to make EEPSAC-GE films show hydrophobic property.

|
Download:
|
| Fig. 4.The contact angles (CAs) of (a) initial gelatin; (b) 2% (ws/wg) DEEPSAC-GE; (c) 6% (ws/wg) DEEPSAC-GE; (d) 2% (ws/wg) EPDDMAC-GE; (e) 6% (ws/wg) EPDDMACGE; (f) 8% (ws/wg) EPDDMAC-GE. | |
The antibacterial of the polymers were investigated by a method based on that of Oraphan et al. [13]. Bacteria,Aspergillus niger,Bacillus subtilis,Staphylococcus aureus and Escherichia coli, were supplied by the Food and Bioengineering College of the Qilu University of Technology of China. As seen from Table 2,the two polymers have no antimicrobial activity against A. niger (belonging to fungi),but have excellent antimicrobial activity against S. aureus,and B. subtilis (both belonging to Gram-positive bacteria) and E. coli (belonging to Gram-negative bacteria). The different bactericidal activity of the polymers is attributed to the structure of the microbial cell [14, 15, 16]. EPDDMAC-GE has proven to have a higher antimicrobial activity than DEEPSAC-GE using the minimum inhibitory concentration (MIC). EPDDMAC is long-chain quaternary ammonium salt,and the longer the chain length,the stronger the ability to dissolve the fat cells of the bacteria and kill the bacteria acceleratively. Fig. 5 is the camera images of inhibition zone of EPDDMAC-GE (1 mg/mL) and DEEPSAC-GE (1 mg/mL) against S. aureus,B. subtilis and E. coli. The sizes of the inhibition zones of the two polymers against E. coli are slightly smaller than against S. aureus and B. subtilis. This can be explained by three reasons. First,the absence of an outer membrane and the presence of negatively charged teichoic acid molecules within a thick peptidoglycan layer (20-80 nm) on the surface of S. aureus should make them more attractive to the positively charged molecules,so S. aureus is more easily damaged by positively charged molecules than E. coli. Second,the presence of a small number of channels of porins within the outer membrane of E. coli may help block the entrance of the polymers into the bacterial cell,making them more difficult to inhibit than S. aureus and B. subtilis. Finally,the smaller dimensions of S. aureus and B. subtilis may partly account for the more intimate contact with the polymers,making EPDDMAC-GE and DEEPSAC-GE have more effective antibacterial activity against S. aureus and B. subtilis than against E. coli [17, 18].
| Table 2 The MIC of EPDDMAC-GE and DEEPSAC-GE. |

|
Download:
|
| Fig. 5.The antibacterial activity of (a) EPDDMAC-GE against S. aureus; (b) EPDDMAC-GE against B. subtilis; (c) EPDDMAC-GE against E. coli; (d) DEEPSAC-GE against S. aureus; (e) DEEPSAC-GE against B. subtilis; (f) DEEPSAC-GE against E. coli. | |
In summary,the chemical structures of EPDDMAC-GE and DEEPSAC-GE were characterized by FT-IR,13C NMR and elemental analysis. The results of water contact angles indicated that EPDDMAC-GE is a hydrophilic molecule,while DEEPSAC-GE is a hydrophobic molecule. The gelatin-modified polymers exhibit excellent antibacterial properties.
AcknowledgmentsThis work was supported by Shandong Provincial Natural Science Foundation (Nos. Y2008B35 and ZR2010BQ002) and the Star Youth Science and Technology Plans Project of Ji’nan City (No. 20110103). We also appreciate the support of Program for Scientific Research Innovation Team in Colleges and Universities of Shandong Province.
| [1] | G. Vargas, J.L. Acevedo, J. López, Study of cross-linking of gelatin by ethylene glycol diglycidyl ether, Mater. Lett. 62 (2008) 3656-3658. |
| [2] | J. Chen, E. Dickinson, Protein/surfactant interfacial interactions. Part 1. Flocculation of emulsions containing mixed protein + surfactant, Colloids Surf. A 100 (1995) 255-265. |
| [3] | S. Farris, J.H. Song, Q.R. Huang, An alternative reaction mechanism for the crosslinking of gelatin with glutaraldehyde, J. Agric. Food Chem. 58 (2010) 998-1003. |
| [4] | L. Guo, R.H. Colby, C.P. Lusignan, A.M. Howe, Physical gelation of gelatin studied with rheo-optics, Macromolecules 36 (2003) 10009-10020. |
| [5] | D. Hellio-Serughetti, M. Djabourov, Gelatin hydrogels cross-linked with bisvinyl sulfonemethyl. 2. The physical and chemical networks, Langmuir 22 (2006) 8516-8522. |
| [6] | J. Xu, T.D. Li, X.L. Tang, C.D. Qiao, Q.W. Jiang, Effect of aggregation behavior of gelatin in aqueous solution on the grafting density of gelatin modified with glycidol, Colloids Surf. B: Biointerfaces 95 (2012) 201-207. |
| [7] | Y. Kamiyama, J. Israelachvili, Effect of pH and salt on the adsorption and interactions of an amphoteric polyelectrolyte, Macromolecules 25 (1992) 5081-5088. |
| [8] | D.D. Van Slyke, A method for quantitative determination of aliphatic amino groups: applications to the study of proteolysis and proteolytic products, J. Biol. Chem. 9 (1911) 185-204. |
| [9] | R.A. Milch, Infra-red spectra of deuterated gelatin sols, Nature 202 (1964) 84-85. |
| [10] | J.E. Sarneski, H.L. Surprenant, F.K. Molen, C.N. Reilley, Chemical shifts and protonation shifts in carbon-13 nuclear magnetic resonance studies of aqueous amines, Anal. Chem. 47 (1975) 2116-2118. |
| [11] | H. Fraenkel-Conrat, The action of 1,2-epoxides on proteins, J. Biol. Chem. 154 (1944) 227-238. |
| [12] | I. Yakimets, N. Wellner, A.C. Smith, et al., Mechanical properties with respect to water content of gelatin films in glassy state, Polymer 46 (2005) 12577-12585. |
| [13] | W. Oraphan, T. Nuttha, K. Suda, P.H. Voravee, Surface-quaternized chitosan particles as an alternative and effective organic antibacterial material, Colloid Surf. B 92 (2012) 121-129. |
| [14] | S. Rostamizadeh, M. Nojavan, R. Aryan, H. Sadeghian, M. Davoodnejad, A novel and efficient synthesis of pyrazolo[3,4-d]pyrimidine derivatives and the study of their anti-bacterial activity, Chin. Chem. Lett. 24 (2013) 629-632. |
| [15] | L.Q. Fu, C.Y. Ling, X.S. Guo, H.L. He, Y.S. Yang, Synthesis and antibacterial activity of pleuromutilin derivatives with novel C(14) side chain, Chin. Chem. Lett. 23 (2012) 9-12. |
| [16] | L.Q. Fu, X.S. Guo, X. Liu, et al., Synthesis and antibacterial activity of C-2(S)-substituted pleuromutilin derivatives, Chin. Chem. Lett. 21 (2010) 507-510. |
| [17] | N. Vallapa, O. Wiarachai, N. Thongchul, et al., Enhancing antibacterial activity of chitosan surface by heterogeneous quaternization, Carbohyd. Polym. 83 (2011) 868-875. |
| [18] | A.M. Bonilla, M.F. García, Polymeric materials with antimicrobial activity, Prog. Polym. Sci. 37 (2012) 281-339. |