b College of Materials and Chemical Engineering, Hainan University, Haikou 570228, China;
c School of Chemical and Material Engineering, Jiangnan University, Wuxi 214122, China
Derivatives of 2,2'-arylmethylene dicyclohexane-1,3-dione, which serve as key intermediates in the synthesis of xanthenes, an important class of biological active compounds,are typically synthesized via the Knoevenagel condensation and Michael addition of aromatic aldehydes with 1,3-cyclohexanedione,or other types of 1,3-cyclic diketones. Jin et al. carried out the condensation by grinding at room temperature [1]. Kantevari and coworkers used HClO4-SiO2 as catalyst to synthesize the title compounds in 44.5%-91.0% yields [2]. In the presence of CsF, Nandre et al. synthesized the target product in 84%-96% yields [3]. Recently,Li et al. reported that the condensation can be catalyzed by urea under ultrasound [4]. However,these reported chemical synthetic methods suffer from a number of drawbacks,such as low yield,harsh reaction conditions and tedious work-up processes. To fulfill the requirements of green chemistry and to simplify the production process,a simple,efficient and eco-friendly method should be developed for the synthesis of 2,2'-arylmethylene dicyclohexane-1,3-dione derivatives.
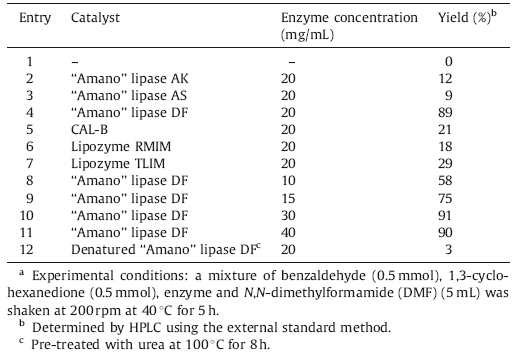
During the last decade,biocatalysis has emerged as an efficient and powerful tool in modern synthetic chemistry due to its valued features,such as high efficiency,mild reaction conditions and lower energy requirements [5]. One current frontier for biocatalysis is enzymatic promiscuity,where enzymes catalyze alternative reactions that differ from their natural physiological reaction. The need for new efficient and eco-friendly catalysts in industry is evident and enzymaticpromiscuity canmeet these requirementswell,which has largelybroadenedtheapplicablerangeofbiocatalysisinthesynthesis of fine chemicals [6]. Examples of enzyme promiscuity-related reactions include Aldol reaction [7],Baylis-Hillman reaction [8] and Mannich reaction [9],while no enzyme catalyzed Knoevenagel- Michael cascade reactions of aromatic aldehydes and 1,3-cyclic diketones have been reported. Among the promiscuous enzymes, lipases,which naturally hydrolyze triglycerides,have attracted much attention due to their excellent features,such aswide sources,broad substrate spectrum and high stability. In continuation of our work [10] on the enzymatic promiscuity,herein we report a novelmethod for the synthesis of 2,2'-arylmethylene dicyclohexane-1,3-dione derivatives using ‘‘Amano’’ lipase DF as a catalyst (Scheme 1).
|
Download:
|
| Scheme 1.‘‘Amano’’ lipase DF-catalyzed synthesis of 2,2'-arylmethylene dicyclohexane-1,3-dione derivatives. | |
Analytical thin layer chromatography (TLC) was performed on Haiyang precoated TLC plates (silica gel GF254),using petroleum ether/ethyl acetate (1/1,v/v) as the elution solution. The 1H NMR (400 MHz) spectra were recorded on a Bruker Advance 2B 400 instrument. Chemical shifts (δ) are quoted in ppm using CDCl3 (1H NMR δ 7.26,13C NMR δ 77.16) as the solvent and tetramethylsilane (TMS) as the internal reference. All reagents wereof analytical grade and used as received without any further purification. HPLC was carried out on a Agilent 1100 HPLC system equipped with a Daicel 19423 CHIRALPAK AD-H column,using n-hexane/isopropanol (90:10,v/v) as the mobile phase at a flow rate of 1.0 mL/min and UV detection at 254 nm. All melting points were determined with a X-4 microscope melting-point testing apparatus and were uncorrected. Thestructures of the productswereconfirmedby comparing the NMR spectral data with those reported in the literature.
General procedure for the synthesis of target compounds (3a- 3m): A mixture of aromatic aldehydes (0.5 mmol),1,3-cyclic diketone (0.5 mmol) and ‘‘Amano’’ lipase DF (20 mg/mL) in N,Ndimethylformamide (DMF) (5 mL) was shaken at 200 rpm,40 ℃ for specified time. Progress of the reaction was monitored by TLC. After completion of the reaction,10 mL cold distilled water was added to the reaction mixture,then extracted with dichloromethane (2×15 mL). The organic phases were combined,concentrated and purified by silica gel column chromatography (petroleum ether:ethyl acetate = 2:1,v/v) or recrystallization from ethanol to give pure product.
Selected data of 3a: White solid; Mp 210-211 ℃; 1H NMR (400 MHz,CDCl3): δ 12.35 (s,1H,OH),12.19-11.88 (m,1H,OH), 8.14-8.05 (m,1H,Ar-H),7.48 (t,1H,J = 7.8 Hz,Ar-H),7.28 (s,0.3H, Ar-H),7.24 (s,0.4H,Ar-H),7.17 (t,1H,J = 7.1 Hz,Ar-H),7.10 (d,1H, J = 8.1 Hz,Ar-H),5.47 (s,1H,CH),2.75-1.52 (m,12H,CH2); 13C NMR (101 MHz,CDCl3): δ 191.82,189.79,138.18,133.70,130.18, 128.50,128.16,126.48,125.85,116.45,33.51,32.99,32.92,20.13; EI-MS m/z 312 [M]+.
Characterization data of other compounds are shown in Supporting information. 3. Results and discussion
Initially,benzaldehyde (1a) and 1,3-cyclohexanedione (2a) were chosen as model substrates to optimize the reaction conditions (Table 1). As expected,no product was observed in the absence of the catalyst (Table 1,entry 1). In the cases of ‘‘Amano’’ lipase AK and ‘‘Amano’’ lipase AS,the target compound 3a was obtained in yields of only 12% and 9%,respectively (Table 1, entries 2 and 3). CAL-B,Lipozyme RMIM and Lipozyme TLIM also showed low catalytic activities in these cascade reactions,giving the target product in yields of 21%,18% and 29%,respectively (entries 5-7,Table 1). When the reactants were incubated with ‘‘Amano’’ lipase DF,a yield of 89% was achieved,which was the highest among all enzymes tested (Table 1,entry 4). As a control experiment,the reactants were incubated with the urea-denatured ‘‘Amano’’ lipase DF,compound 3a was obtained only in 3% yield, indicating the reaction was indeed catalyzed by the biologically active ‘‘Amano’’ lipase DF (Table 1,entry 12).
| Table 1 Optimization of the reaction conditions for the synthesis of 3a.a |
Since enzyme concentration often plays an important role in enzymatic reactions,concentrations of ‘‘Amano’’ lipase DF were tested in the range of 10-40 mg/mL. As shown in Table 1,the yield of the target product was evidently improved when the enzyme concentration increased from 10 mg/mL to 20 mg/mL (Table 1, entries 4,8 and 9). However,further increase in the enzyme concentration only led to slight change in the yield (Table 1,entries 10 and 11),suggesting that 20 mg/mL was a suitable concentration for the catalysis of the cascade reactions.
With the optimal reaction conditions in hand,a series of aromatic aldehydes and 1,3-cyclic diketones were investigated to test the generality and scope of this ‘‘Amano’’ lipase DF-catalyzed Knoevenagel-Michael cascade reactions. The results are shown in Table 2. Aromatic aldehydes bearing both electron-donating and electron-withdrawing groups formed corresponding 2,2'-arylmethylene dicyclohexane-1,3-dione derivatives in good to excellent yields (Table 2,entries 1-13). Among the factors influencing the reaction rate,the substituent position on the benzene ring of aromatic aldehydes played an important role. The substituent in the ortho-position led to a longer reaction time compared to that in the para-position,which was most likely due to steric hinderance (entry 12 vs. entry 13,Table 2). In addition,longer reaction time were required with 5,5-dimethyl-1,3-cyclohexanedione in comparison to 1,3-cyclohexanedione,which can also be attributed to steric hinderance (entries 1-5 vs. entries 6-13,Table 2).
| Table 2 Synthesis of compound 3a-3m by lipase DF in DMF. |
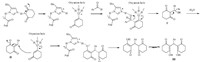
Based on these experimental results and the widely accepted viewpoint that the hydrolysis active site in lipase is responsible for its promiscuous performance [11],a mechanism is proposed and depicted in Scheme 2. Firstly,1,3-cyclic diketone was activated by the Asp-His dyad to form an enolate anion,which was stabilized by the oxyanion hole of the lipase. Then the enolate anion attacked the aromatic aldehyde to form the intermediate Ⅰ. Subsequently, dehydration took place to generate the intermediate Ⅱ. Finally, Michael addition occurred when another enolate anion attacked the intermediate Ⅱ and gave the product Ⅲ.
|
Download:
|
| Scheme 2.Proposed mechanism for ‘‘Amano’’ lipase DF-catalyzed synthesis of 2,2'-arylmethylene dicyclohexane-1,3-dione derivatives. | |
In summary,we have developed a novel method for the enzymatic synthesis of 2,2'-arylmethylene dicyclohexane-1,3- dione derivatives via Knoevenagel-Michael cascade reactions catalyzed by ‘‘Amano’’ lipase DF in anhydrous media. High yields, mild reaction conditions,simple work-up procedure and ecofriendliness are the features of this new protocol with great potential in industrial applications.
AcknowledgmentsThis work was financially supported by the Outstanding Young Scholar Grant of Zhejiang University (No. R4110092),the National Natural Science Foundation of China (No. 21176215/21176102) and the Program for Zhejiang leading team of S&T Innovation (No. 2011R50007). We thank all of the constructive comments and suggestions from the editors and reviewers.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.04.007.
| [1] | T.S. Jin, J.S. Zhang, A.Q. Wang, T.S. Li, Solid-state condensation reactions between aldehydes and 5,5-dimethyl-1,3-cyclohexanedione by grinding at room temperature, Synth. Commun. 35 (2005) 2339-2345. |
| [2] | S. Kantevari, R. Bantu, L. Nagarapu, HClO4-SiO2 and PPA-SiO2 catalyzed efficient one-pot Knoevenagel condensation, Michael addition and cyclo-dehydration of dimedone and aldehydes in acetonitrile, aqueous and solvent-free conditions: scope and limitations, J. Mol. Catal. A: Chem. 269 (2007) 53-57. |
| [3] | K.P. Nandre, V.S. Patil, S.V. Bhosale, CsF mediated rapid condensation of 1,3-cyclohexadione with aromatic aldehydes: comparative study of conventional heating vs. ambient temperature, Chin. Chem. Lett. 22 (2011) 777-780. |
| [4] | J.T. Li, Y.W. Li, Y.L. Song, G.F. Chen, Improved synthesis of 2,20-arylmethylene bis(3-hydroxy-5,5-dimethyl-2-cyclohexene-1-one) derivatives catalyzed by urea under ultrasound, Ultrason. Sonochem. 19 (2012) 1-4. |
| [5] | C.C.C.R. de Carvalho, Enzymatic and whole cell catalysis: finding new strategies for old processes, Biotechnol. Adv. 29 (2011) 75-83. |
| [6] | M.S. Humble, P. Berglund, Biocatalytic promiscuity, Eur. J. Org. Chem. 19 (2011) 3391-3401. |
| [7] | Z.B. Xie, N. Wang, L.H. Zhou, et al., Lipase-catalyzed stereoselective cross-aldol reaction, ChemCatChem 5 (2013) 1935-1940. |
| [8] | M.T. Reetz, R. Mondière, J.D. Carballeira, Enzyme promiscuity: first protein-catalyzed Morita-Baylis-Hillman reaction, Tetrahedron Lett. 48 (2007) 1679-1681. |
| [9] | S.J. Chai, Y.F. Lai, H. Zheng, P.F. Zhang, A novel trypsin-catalyzed three-component Mannich reaction, Helv. Chim. Acta 93 (2010) 2231-2236. |
| [10] | L. Jiang, H.W. Yu, An example of enzymatic promiscuity: the Baylis-Hillman reaction catalyzed by a biotin esterase (BioH) from Escherichia coli, Biotechnol. Lett. 36 (2014) 99-103. |
| [11] | C. Branneby, P. Carlqvist, K. Hult, T. Brinck, P. Berglund, Aldol additions with mutant lipase: analysis by experiments and theoretical calculations, J. Mol. Catal. B: Enzym. 31 (2004) 123-128. |