b School of Chemistry and Chemical Engineering, Anhui University, Hefei 230039, China
Rapid development in the field of catalysis has directed increasing attention on the catalyst. Phase-transfer catalysis and micellar phase-transfer catalysis are two well-known methods of promoting organic reactions,but surfactants are among the most important catalysts. Traditional single-chained surfactants have been widely studied [1, 2, 3]. In 1991,Mengeret al.synthesized,for the first time,reported on a series of three,two-chained surfactants with lower critical micell concentration (CMC) than traditional,single-chained surfactants and named them gemini surfactants. In recent years,more and more investigators are studying these new structural surfactants and their phasetransfer catalytic effects [4, 5, 6, 7, 8, 9, 10, 11]. However,a few researchers have studied the micellar catalysis of gemini surfactants [12, 13, 14, 15, 16, 17]. For example,Qiu et al. [13] studied micellar-catalyzed alkaline hydrolysis of 2,4-dinitrochlorobenzene (DNCB) in the presence of the cationic gemini surfactant,1,2-ethane bis(dimethyldodecyl ammonium bromide),and found that this system had a relatively low second-order rate constant in micellar pseudophase and a great deviation from the pseudophase ion-exchange model at high concentration. Also some studies examined aqueous/organic two-phase reactions [18, 19] or focused on studying their elemental characteristics,for example,since they can be more easily adsorbed in the interface between water and air at lower critical micelle concentrations [4],provide greater efficiency in reducing the surface tension and lowering the Krafft temperature, and better solubilization in comparison with conventional surfactants [20, 21, 22, 23, 24].
Until now,it was reported [1, 25, 26, 27, 28] that extensive micellar catalytic reactions were generally carried out in solution with conventional monomeric surfactants. However,still few studies involved liquid-solid catalysis effects on inorganic/organic reactions using monomeric surfactants,let alone gemini surfactants.
In this paper,a new gemini surfactant connecting two alkyldimethylammonium bromide moieties,namely 12-10-12, was synthesized and mainly applied to catalysis reactions. Two synthetic steps were designed to form the final product which had a flexible spacer containing ten carbon atoms and two alkyl chains with twelve carbon atoms (Scheme 1). Moreover,the catalysis effect on the reaction of anhydrous sodium acetate and 4-methylbenzyl chloride was also investigated,in contrast to the traditional monomeric surfactants. The results show that 12-10-12 possesses better catalysis ability. Based on the related knowledge,a theoretical model was presented to explain why this gemini surfactant could promote liquid-solid reactions.

|
Download:
|
| Scheme 1. Synthetic route for the gemini surfactant 12-10-12. | |
2.1. Materials and methods
Chemicals,reagents and solvents from commercial sources are of analytical or spectroscopy grade and used as received without further purification. 1H NMR spectra were recorded on an AVANCE-400 NMR spectrometer. Elemental analyses were carried out on an Elementar Vario EL-•. FT-IR spectra were measured on a Nicolet NEXUS 870 spectrometer (KBr discs). Thermogravimetry analyses (TGA) were carried out using a Pyris1 TGA (PE Corp.,USA) at a heating rate of 10°C/min from 40°C to 600°C under a nitrogen atmosphere. 2.2. Synthesis and characterization
Synthesis of compound A: Compound A was obtained by refluxing dimethyl hexanedioate (8.70 g,50.00 mmol) with dimethylaminoethanol (26.70 g,300.00 mmol) in the presence of 1.50 g dibutyltin dilaurate catalyst at 100°C for 6 h (Scheme 1). The by-products of methanol and residual dimethylaminoethanol were removed by vacuum from the reaction mixture and the crude product was purified by column chromatography. Yield: 9.00 g (62.50%).1H NMR (400 MHz,CDCl3 ): δ 1.66-1.73 (m,4H, CCH2CH2C),2.33-2.56 (m,4H,2×COCH2),3.48 (s,12H, 4×CH3),4.14-4.17 (t,4H,J= 7.2 Hz,2×NCH2),4.46-4.72 (m, 4H,OCH2). IR (KBr,cm-1): 2947,2822,1736,1460,1383,1172, 1041 [15].
Synthesis of 12-12-12: A solution of compound A (5.00 g,17.40 mmol) andn-C12H25Br (13.00 g,52.50 mmol) in acetone was refluxed for 24 h. The precipitate was recrystallized from acetone to afford white crystals 12.50 g. Yield: 91.60%.1H NMR (400 MHz,CDCl3 ):δ0.86-0.88 (t,6H,J= 6.8 Hz,2×CH3),1.25-1.36 (m,36H,2×(CH2)9),1.71-1.77 (m,8H,2×CH2,CCH2CH2C),2.33-2.56 (m,4H,2×CH2COO),3.48 (s,12H,2×(CH3)2),3.62-3.66 (t,4H, J= 7.6 Hz,2×2CH2),4.14-4.17 (t,4H,J= 7.2 Hz,2×CH2N+),4.47- 4.73 (m,4H,2×CH2) [29]. Anal. calcd. for C38H78Br2N2O4: C,57.98; H,9.92; N,3.56. Found: C,57.37; H,9.94; N,3.53. IR (KBr,cm-1):2921,2851,1723,1472,1382,1274,1153. 2.3. Kinetic measurements
Anhydrous sodium acetate,3.50 g (42.70 mmol,0.9163 mmol/L),4-methylbenzyl chloride 5.00 g (35.60 mmol, 0.764 mol/L) and a certain amount of 12-10-12were added to 40 mL DMF. The mixture was stirred at required speed and desired temperature. The reaction continued for 7 h. The samples were collected at regular intervals for analysis using Agilent 7820 GasChromatograph equipped with a FID detector,and orthodichlorobenzene served as an internal standard. The 4-methylbenzyl acetate was extracted by benzene.
The kinetic equations are expressed as follows:
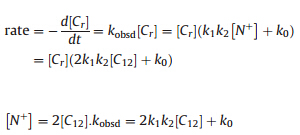
where kobsd is the apparent first-order rate constant for the reaction,[Cr] is the concentration of 4-methylbenzyl chloride,[C12] is the concentration of 12-10-12,k0 is the first-order rate constant without phase transfer catalyst,k1is the equilibrium constant and k2 is the second-order-rate constant with the phase transfer catalyst,respectively. The above equations suggest: (1) The reaction is first-order with respect to the concentration of 4-methylbenzyl chloride and the plots of ln[Cr] vs.time should be linear. (2) kobsdincreases with the concentration of phase transfer catalyst and the plots of kobsdvs.[C12] are linear lines. 3. Results and discussion 3.1. Thermal analysis
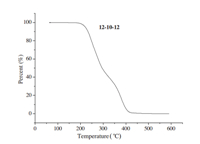
The TGA measurement of 12-10-12was determined in the range of 20-600°C under nitrogen (Fig. 1). In order to study the thermal stability of this compound,thermogravimetric analysis (TGA) was performed on single crystal samples. TGA data show that 12-10-12is stable up to 205°C,loses weight from 205°Cto 320°C corresponding to decomposition of double alkyl chains,and finally loses weight from 320°C to 410°C corresponding to the losses of the flexible spacer.
|
Download:
|
| Fig. 1. TGA curve of 12-10-12. | |
According to previous knowledge [13, 24],a model to explain the liquid-solid phase transfer catalysis reaction mechanism has been proposed for the nucleophilic substitution reaction of anhydrous sodium acetate and 4-methylbenzyl chloride with the phase transfer catalyst of 12-10-12(Scheme 2). It is noted that the solubility of anhydrous sodium acetate in DMF is poor at 60°C.Some kinetic and thermodynamic parameters to better understand the catalytic mechanism were obtained. The reaction of 4-methylbenzyl chloride and anhydrous sodium acetate with the participation of 12-10-12can be supposed to take place in the liquid-solid interface.
|
Download:
|
| Scheme 2. Model of solid–liquid phase transfer catalysis reaction mechanism (k0 is the first-order rate constant without phase transfer catalyst,k1 is the equilibrium constant and k2 is the second-order-rate constant with the phase transfer catalyst, respectively). | |
As represented by the reaction mechanism of the micellar catalysis in Scheme 2,the hydrophilic group of gemini surfactant initially unites with CH3COO- group,and then reacts with 4-methylbenzyl chloride through the nucleophilic substitution reaction. While the increasing of aggregation number and the micelle size may significantly influence the reaction rate between the reactants at the micelle surface region. So when the concentration of 12-10-12 approaches the value of CMC,the molecules of the gemini surfactant began to form micelles (molecular agglomerates),and the number of micelles will increase with the concentration of 12-10-12.By this stage,the cationic gemini surfactants can bind with more and more CH3COO- groups at the micelle surface region. This is to the benefit of the process of the nucleophilic substitution reaction,and thus leads to the increasing of the reaction rate. 3.3. Influence of surfactant concentration
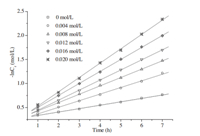
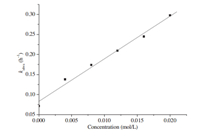
Firstly,in order to investigate the effect of surfactant concentration for the apparent-first-order rate constants ( kobsd), a series of nucleophilic reactions was carried out in the presence of 12-10-12with various concentrations at 50°C. As shown by the -lnCr vs.time characteristics in Fig. 2,all the kinetic results fit the first-order rate equation very well: ln(1/Cr)= kobsd t+A. The apparent first-order rate constants ( kobsd) and linear correlation coefficients were evaluated from the linear plots of ln(1/Cr)vs.time. The data are summarized in Table 1. Clearly, the values of linear correlation coefficients were close to one, which indicated that the association between ln(1/Cr)and reaction time is coincident with a linear relationship and the experimental results were reliable. Then,Fig. 3 was obtained by using the values of the concentrations of 12-10-12(C12)andthe apparent first-order rate constants ( kobsd)inTable1astheXand Y axes,respectively. The relationship between the apparent first-order rate constants ( kobsd) and the concentrations of 12-10-12(C12) was also close to a linear relationship,and its slope and intercept were estimated to be 10.6 and 0.071,respectively. Finally,the linear equation between the surfactant concentration (C12) and the apparent-first-order rate constants ( kobsd)is deduced as follows:
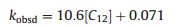
|
Download:
|
| Fig. 2. Plot of -lnCr vs.time for the phase transfer catalysis by 12-10-12 at 50°C. [4-Methylbenzyl chloride] = 0.764 mol/L, [sodium acetate] = 0.9163 mol/L. | |
|
Download:
|
| Fig. 3. Plot ofkobsdvs.concentration of 12-10-12 for the phase transfer catalysis by 12-10-12 at 50°C. [4-Methylbenzyl chloride] = 0.764 mol/L, [sodium acetate] = 0.9163 mol/L. | |
In addition,the effect of the concentration of 4-methylbenzyl chloride on the reaction rate was also studied,and a series of nucleophilic reactions was carried out in the presence of 12-10-12 at fixed concentrations at 50°C. As shown in Fig. S1 (Supporting information),although the concentration of 4-methylbenzyl chloride is different,the ratios between the values of -lnCr and reaction time (h) are almost same,which indicates the concentration of 4-methylbenzyl chloride can influence the reaction rate,and it does not impact the apparent first-order rate constants ( kobsd). 3.4. Influence of reaction temperature
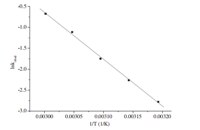
The nucleophilic substitution reaction was carried out at various temperatures (at 40,45,50,55 and 60°C) when the concentration of 12-10-12was 8.00 mmol/L (Fig. 4). The influence of temperature on -lnCr was studied according to the Arrhenius equation: ln kobsd= -Ea/RT+lnA. Fig. 5 gave the plot of ln kobsdvs.1/ T. The apparent activation energy was 93.11 kJ/mol obtained by the slope.

|
Download:
|
| Fig. 4. Plot of -lnCr vs. time for phase transfer catalysis by 12-10-12at different temperatures. [4-Methylbenzyl chloride] = 0.764 mol/L, [sodium acetate] = 0.9163 mol/L. | |
|
Download:
|
| Fig. 5. Plot of lnkobsd vs. 1/T for phase transfer catalysis by 12-10-12. [4-Methylbenzyl chloride] = 0.764 mol/L, [sodium acetate] = 0.9163 mol/L. | |
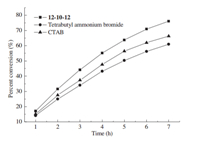
The nucleophilic substitution reaction was studied at 50°C when the concentration of surfactants was 12.00 mmol/L. The surfactants were 12-10-12,tetrabutyl ammonium bromide and hexadecyl trimethyl ammonium bromide (CTAB). It is evident from Figs. 6 and 7 that the catalysis kinetic model of 12-10-12was similar to that of conventional quaternary ammonium salt catalyst and the catalytic activity of 12-10-12was more efficient than tetrabutyl ammonium bromide and CTAB at the same concentration. The reaction rate with 12-10-12was greater than those of tetrabutyl ammonium bromide and CTAB,which can be explained in view of unique molecular structure of the gemini surfactant with two catalyst active sites (active site is meant to be the catalyst cation N+) in one molecule.

|
Download:
|
| Fig. 6. Plot of -lnCr vs.time for phase transfer catalysis by 12-10-12, tetrabutyl ammonium bromide and CTAB, respectively. [4-Methylbenzyl chloride] = 0.764 mol/L, [sodium acetate] = 0.9163 mol/L, [12-10-12] = 0.012 mol/L, [tetrabutyl ammonium bromide] = 0.012 mol/L, [CTAB] = 0.012 mol/L. | |
|
Download:
|
| Fig. 7. Plot of percent conversionvs.time for phase transfer catalysis by 12-10-12 and tetra butyl ammonium bromide, respectively. [4-Methylbenzyl chloride] = 0.764 mol/L, [sodium acetate] = 0.9163 mol/L, [12-10-12] = 0.012 mol/L, [tetrabutyl ammonium bromide] = 0.012 mol/L, [CTAB] = 0.012 mol/L. | |
In summary,a gemini surfactant ( 12-10-12)with ester groups was conveniently synthesized and an investigation of the liquidsolid phase transfer reaction of anhydrous sodium acetate and 4-methylbenzyl chloride in the presence of 12-10-12 has been studied. It was found that the phase transfer catalyst of gemini surfactant ( 12-10-12) was more effective than the traditional surfactant phase transfer catalyst (such as tetrabutyl ammonium bromide). A suitable kinetic model was proposed to explain the observed experimental results and it was determined that the experimental results fit the model very well. Acknowledgment
This work was supported by the Talent Foundation of Anhui Science and Technology University (No. ZRC2014401). Appendix A. Supplementary data
Supplementary material related to this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.04.006.
| [1] | E. Pandey, S.K. Upadhyay, Effect of micellar aggregates on the kinetics of oxidation of α-aminoacids by chloramine-T in perchloric acid medium, Colloids Surf. A: Physicochem. Eng. Asp. 269 (2005) 7-15. |
| [2] | X.H. Zhao, Z.W. Ye, A facile synthesis of a novel energetic surfactant1-amino-3-dodecyl-1,2,3-triazolium nitrate, Chin. Chem. Lett. 25 (2014) 209-211. |
| [3] | H.Y. Fu, M. Li, H. Chen, et al., Higher olefin hydroformylation in organic/aqueous biphasic system accelerated by double long-chain cationic surfactants, J. Mol. Catal. A: Chem. 259 (2006) 156-160. |
| [4] | R. Zana, M. Benrraou, R. Rueff, Alkanediyl-α,ω-bis(dimethylalkylammonium bromide)surfactants. 1. Effect of the spacer chain length on the critical micelle concentration and micelle ionization degree, Langmuir 7 (1991) 1072-1075. |
| [5] | F.M. Menger, C.A. Littau, Gemini surfactants: a new class of self-assembling molecules, J. Am. Chem. Soc. 115 (1991) 10083-10090. |
| [6] | Q. Xu, L.Y. Wang, F.L. Xing, Synthesis and properties of dissymmetric gemini surfactants, J. Surfact. Deterg. 14 (2011) 85-90. |
| [7] | C.J. Kuo, L.H. Lin, Preparation and properties of new ester-linked cleavable gemini surfactants, J. Surfact. Deterg. 14 (2011) 195-201. |
| [8] | K. Kuperkar, J. Modi, K. Patel, Surface-active properties and antimicrobial study of conventional cationic and synthesized symmetrical gemini surfactants, J. Surfact. Deterg. 15 (2012) 107-115. |
| [9] | N. Azum, A.M. Asiri, M.A. Rub, Mixed micellization of gemini surfactant with nonionic surfactant in aqueous media: a fluorometric study, Colloid J. 75 (2013) 235-240. |
| [10] | T. Yoshimura, T. Ichinokawa, M. Kaji, et al., Synthesis and surface-active properties of sulfobetaine-type zwitterionic gemini surfactants, J. Colloids Surf. A: 273 (2006) 208-212. |
| [11] | A.R. Tehrani-Bagha, H. Oskarsson, C.G. van Ginkel, et al., Cationic ester-containing gemini surfactants: chemical hydrolysis and biodegradation, J. Colloid Interface Sci. 312 (2007) 444-452. |
| [12] | H.E. Ali, Cycloalkylation reactions of fatty amines with a,v-dihaloalkanes: role of bis-quaternary ammonium salts as phase-transfer catalysts, Catal. Commun. 8 (2007) 855-860. |
| [13] | L.G. Qiu, A.J. Xie, Y.H. Shen, Micellar-catalyzed alkaline hydrolysis of 24-dinitrochlorobenzene in a cationic gemini surfactant, J. Colloids Surf. A. 260 (2005) 251-254. |
| [14] | G.D. Yadav, C.K. Mistry, Oxidation of benzyl alcohol under a synergism of phase transfer catalysis and heteropolyacids, J. Mol. Catal. A. 172 (2001) 135-149. |
| [15] | A. Bendjeriou-Sedjerari, G. Derrien, C. Charnay, et al., Contribution of 1H NMR to the investigation of the adsorption of cationic Gemini surfactants with oligooxyethylene spacer group onto silica, J. Colloid Interface Sci. 331 (2009) 281-287. |
| [16] | T.M. Zubareva, A.V. Anikeev, E.A. Karpichev, Catalysis of the alkaline hydrolysis of 4-nitrophenyl diethyl phosphonate by cationic dimeric surfactant micelles, Theor. Exp. Chem. 47 (2011) 108-114. |
| [17] | G. Cerichelli, L. Luchetti, G. Mancini, G. Savelli, Cyclizations of 2-(v-bromoalkyloxy) phenoxide ions in dicationic surfactants, Langmuir 15 (1999) 2631-2634. |
| [18] | W.A. Herrmann, C.W. Kohlpaintner, Water-soluble ligands, metal complexes, and catalysts: synergism of homogeneous and heterogeneous catalysis, Angew. Chem. Int. Ed. Engl. 32 (1993) 1524-1544. |
| [19] | B. Cornils, Bulk and fine chemicals via aqueous biphasic catalysis, J. Mol. Catal. A 143 (1999) 1-10. |
| [20] | M. In, V. Bec, O. Aguerre-Chariol, R. Zana, Quaternary ammonium bromide surfactant oligomers in aqueous solution: self-association and microstructure, Langmuir 16 (2000) 141-148. |
| [21] | R. Zana, Dimeric and oligomeric surfactants. Behavior at interfaces and in aqueous solution: a review, Adv. Colloid Interface Sci. 97 (2002) 205-253. |
| [22] | P.A. Koya, K. Kabir-ud-Din, Ismail, Micellization and thermodynamic parameters of butanediyl-1,4-bis(tetradecyldimethylammonium bromide) gemini surfactant at different temperatures: effect of the addition of 2-methoxyethanol, J. Sol. Chem. 41 (2012) 1271-1281. |
| [23] | X.J. Xu, J.W. Guo, X. Zhong, Synthesis and properties of novel cationic gemini surfactants with adamantane spacer, Chin. Chem. Lett. 25 (2014) 367-369. |
| [24] | L.G. Qiu, M.J. Cheng, A.J. Xie, Y.H. Shen, Study on the viscosity of cationic gemini surfactant-nonionic polymer complex in water, J. Colloid Interface Sci. 278 (2004) 40-43. |
| [25] | C.A. Bunton, L. Robinson, Micellar effects upon nucleophilic aromatic and aliphatic substitution, J. Am. Chem. Soc. 90 (1968) 5972-5979. |
| [26] | P.D. Burgo, E. Junquera, E. Aicart, Mixed micellization of a nonionic-cationic surfactant system constituted by n-octyl-b-D-glucopyranoside/dodecyltrimethylammonium bromide/H2O. An electrochemical, thermodynamic, and spectroscopic study, Langmuir 20 (2004) 1587-1596. |
| [27] | M.L. Gall, J. Lelièvre, A. Loppinet-Serani, P. Letellier, Thermodynamics and kinetics in micellar media. Reaction of the hydroxide ion with 1,3,5-trinitrobenzene in aqueous solutions of a neutral nonionic surfactant. Effect of the concentration of background electrolyte, J. Phys. Chem. B 107 (2003) 8454-8461. |
| [28] | Q.F. Liu, M. Lu, W. Wei, Chloromethylation of 2-chloroethylbenzene catalyzed by micellar catalysis, Sci. China B: Chem. 52 (2009) 893-899. |
| [29] | M. Li, H.Y. Fu, M. Yang, et al., Micellar effect of cationic gemini surfactants on organic/aqueous biphasic catalytic hydroformylation of 1-dodecene, J. Mol. Catal. A: Chem. 235 (2005) 130-136. |




