b Clinical Division, School of Chinese Medicine, Hong Kong Baptist University, Hong Kong, China;
c Institute of Molecular Functional Materials, Department of Chemistry and Institute of Advanced Materials, Hong Kong Baptist University, Hong Kong, China;
d College of Life and Environmental Sciences, Minzu University of China, Beijing 100081, China;
e HKBU Institute of Research and Continuing Education, Shenzhen Virtual University Park, Shenzhen 518057, China
According to the World Commission on Environment and Development,sustainable development is purported to fulfill the human requirements while protecting the natural environments [1]. Natural resources should be utilized to satisfy the needs of future generations. In textile industries,one of the major challenges is the development of innovative and advanced products. Chemical compounds are favorable to enhance the functionality in dyeing and finishing processes. Among various molecules,heterocyclic aromatic compounds are extensively present in ambient environments including soil,air andwater pollutants,aswell as in animal and plant tissues [2]. A heterocyclic compound is referred to a ring system in which at least one or more carbon atoms are replaced by oxygen, nitrogen or sulphur [3]. Quinoline is one of the more significant heterocyclic compounds [4]. It is regarded as an essential structural element for the synthesis of synthetic dyestuffs because of its relatively high stability and ease of preparation.Quinoline is generally in yellow color and it is commonly used for pharmaceuticals, polymers,anti-oxidation,rubber industry,aswell as the coloration of fibers,fabrics,leathers,paper,wood,detergents,fertilizers,inks, cosmetic products and food [5]. Quinoline derivatives exhibit many biological activities including anticancer,anti-HIV,antimicrobial, antiproliferative,antimycobacterial,anticoccidial,insecticidal,antidyslipidemic and antioxidative properties [6]. Quinoline is also considered as an attractive fluorescent compoundwith high quantum yield and therefore,a number of quinoline-based dyestuffs and colorants has been developed in previous studies [4, 7, 8, 9]. Although quinoline exhibits many desired features in coloration industries,it does process the chondrotoxicity and phototoxicity [10]. Recently, Chan et al. reported a quinoline derivative with anti-tumor activity. However,this compound did not show significant selectivity between cancer cells and normal cells [11]. In the present study,the heteroaromatic dyes were prepared with quinoline compounds and the synthesized dyes were used for the fabric dyeing. The color fastness and the cytotoxicity toward HaCaT skin keratinocytes of the dyed textile samples were evaluated. 2. Experimental 2.1. Synthesis of alkoxy-substituted quinaldine,8-(4-chlorobenzyloxy)- 2-methylquinoline (1a)
Compound 1a was synthesized from compound 1 that was purchased from Sigma-Aldrich,as shown in Scheme 1. A mixture of hydroxyl-substituted quinoline,8-hydroxyl-substituted methylquinoline (1,3 mmol),alkyl halide (RX,3 mmol,where X = Br or Cl) and K2CO3 in 10 mL DMF was stirred at room temperature and the progress was monitor by TLC analysis. After the reaction was complete,the mixture was treated with aqueous Na2CO3 and extracted with chloroform and then dried over anhydrous sodium sulfate. The solvent was removed under reduced pressure and the crude product was purified by silica gel column chromatography to give the pure product. Yield 90.5%; Mp 118.7-119 ℃; 1H NMR (500 MHz,CDCl3): d 2.80 (s,3H),5.41 (s, 2H),6.96 (d,1H,J = 6.5 Hz),7.27-7.36 (m,5H),7.45 (d,2H, J = 8.5 Hz),8.01 (d,1H,J = 8.5 Hz); 13C NMR (125 MHz,CDCl3): d 26.01,70.35,110.76,120.36,122.86,125.70,128.01,128.52, 128.96,133.64,136.01,136.34,140.27,153.82,158.52; HRMS (ESI): calcd. for C17H15NOCl [M+H]+: 284.0842; found: 284.0841.
6-Methoxy-2-methylquinoline (2) was purchased from Sigma- Aldrich. 2.2. UV-visible and fluorescent characterization
Synthesized compounds were dissolved in dichloromethane and adjusted to a concentration at 10-5 mol/L. The solutions were measured with a Perkin Elmer Lambda 35 UV-visible spectrometer and a Perkin Elmer LS35 fluorescence spectrometer. 2.3. Dyeing procedure of acrylic fabrics
Ten milligrams of quinoline compound was firstly dissolved in acetone as a stock solution. The acrylic fabric was dyed with the dye bath containing 1% of quinoline compound in a sample to a liquor ratio of 1:40. After 5 min,1% (v/v) acetic acid and 10% (v/v) sodium sulfate in the total volume of the dye bath were added and the bath was incubated for a further 5 min at room temperature. The bath was then heated to 80 ℃ for 20 min and the temperature was further increased to 97 ℃ for another 45 min. Afterwards,the dyed sample was rinsed with deionised water for several times to remove the non-dyed residuals. The dyed acrylic specimen was then dried at room temperature before being preconditioned in (65 ± 2)% relative humidity at a temperature of 20 ± 2 ℃ for 48 h before evaluation [12]. 2.4. Evaluation of color fastness to washing and light
Color fastness of tested samples to washing was examined using the standard test method AATCC 61-2003 [13]. A specimen with the size of 5 × 10 cm2 and an AATCC multifibre fabric were sewn together and immersed into a washing solution containing 0.15% (w/w) detergent and 50 steel balls. The samples were washed in a Launder-Ometer washing machine for 45 min at 49 ℃. Color fastness of tested specimens to light was determined in accordance with the standard method AATCC 16-2004 [14]. Specimens with 1 × 4 cm2 in size were half covered and placed in an apparatus (Xenotes Alphalm,China) containing xenon arc light for 20 h. For the color change and staining of the conditioned specimens,the original and tested specimens,or original unstained multi-fiber fabrics and tested ones were placed side by side on the same surface inside the light box. D65 artificial daylight and UV light were used on the surface at approximately 458. Gray scale was used to record the grades in color change and staining. 2.5. Cytotoxicity evaluation
HaCaT skin keratinocytes were removed from the sterile cell culture flasks with trypsin and neutralized with fetal bovine serum. After washing with phosphate buffered saline,skin cells were re-suspended in the cell culture medium at a concentration of approximately 1 × 105 cells/mL. Skin cells seeded in the 24 wells plates for 24 h were prepared. Dyed fabricswith 1 mLof 300 μmol/L 1a and 2 were incubated with cells for a further period of 48 h. Doxorubicin (4 mmol/L) was used as a positive control while blank acrylic fabric was used as a negative control. The evaluation of possible toxicity was performed by the sulforhodamine B protein staining methods. Skin cells were fixed with trichloroacetic acid, washed with deionised water and stained with sulforhodamine B. Skin cells were then washed again with acetic acid. Finally,optical images of tested sampleswere captured and the optical absorptions were measured at 575 nm [15]. 3. Results and discussion 3.1. UV-visible and fluorescent characterization
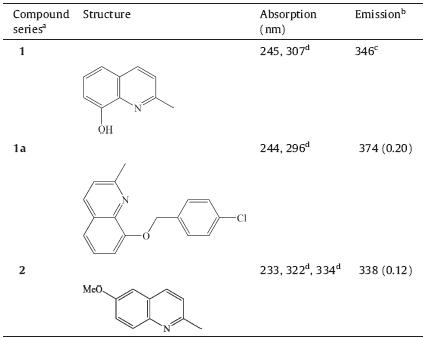
The chemical structures and photophysical properties of the quinoline compounds are shown in Table 1 while the UV-visible spectra of these compounds are illustrated in Fig. 1 Both 1a and 2 possessed a noticeable fluorescent property,bearing the emission at 374 nm with intensity of 0.20 and the emission at 338 nm with intensity of 0.12,respectively. However,other compounds did not exhibit the obvious fluorescent feature as they were only detected to have a very weak emission. Therefore,the two compounds including 1a and 2 were applied for the textile fabrics in order to investigate the fluorescent property of the quinoline compoundstreated textile substrates.
| Table 1 Photophysical data for quinoline derivatives. |

|
Download:
|
| Fig. 1.UV-vis spectrum of a series of quinoline compounds,compound 1 (a),1a (b) and 2 (c). | |
Fig. 2 shows that both compound 1a- and 2-dyed acrylic fabrics did possess an obvious fluorescent effect under UV light when compared with the control fabrics. It was noticed that 1a-dyed sample visibly displayed a higher fluorescent feature than the 2-dyed one. Based on the gray scale shown in Table 2,1a- and 2- dyed acrylic samples resulted in a slight to negligible color change to washing (gray scale = 4-5) and no color staining of adjacent multifibre fabric after washing (gray scale = 5). Both dyed samples showed a fluorescent effect under UV light after washing. Therefore,the color fastness to washing of both dyed acrylic fabric was determined to be good. This might reveal that the basic nitrogen atom in compound 2 might be strongly fixed into amorphous region of acrylic fiber through electrostatic forces. Even under normal laundering at medium temperature,the compound 2 was less prone to leak out to stain on adjacent fabric. After exposed to Xenon arc lamp for 20 h in the Fadeometer,the rating of 1a-dyed acrylic fabric was 3,revealing a fair colourfastness to light while 2- dyed fabric was ranked as 5,indicating a good colourfastness to light when compared to the Blue Wool Standard in the color matching cabinet under the daylight source,D65. Under the ultraviolet source,1a-dyed strip was unable to emit fluorescence whereas 2-dyed strip still displayed a fluorescence character. The results revealed that the photo-stability of compound 1a was considered fair. The light energy might provide a sufficient amount of activation energy for compound 1a to dissociate and change to brownish yellow color. Specifically,the chlorine atom might be easily displaced in free radical reactions during photo-degradation. Thus the molecular structure of 1a might be disrupted and the color of dyed substrate would be adversely affected. However,2 might be less prone to undergo photo-decomposition under visible light. 2 would be more photo-stable since compared to 1a, compound 2 possessed lower molecular weight to have lower chance of light damage. It was noticed that the strongly fixed fiber could be highly hydrophobic that the moisture and oxygen could not be able to enter the fiber,leading to no fading reaction.
| Table 2 Color fastness of 1a and 2 dyed acrylic fabrics to washing and light. |

|
Download:
|
| Fig. 2.(a) 2 dyed acrylic,(b) 1a dyed acrylic and (c) blank acrylic (right) under UV light. | |
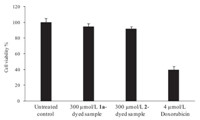
Fig. 3 shows the cytotoxic effect of both compounds (1a) and (2) toward skin keratinocytes. The results revealed that both compounds-dyed samples-treated skin cells did exhibit a high integrity of cellular structure as untreated control whereas cells incubated with the positive control (4 mmol/L doxorubicin) showed a high level of cell condensations and damages. Fig. 4 shows the cell viability of 1a- and 2-dyed fabrics toward HaCaT skin cells. It was observed that both 1a- and 2-dyed acrylic samples did not possess significant cytotoxicity toward skin keratinocytes, with more than 90% of cell viability retained after 48 h treatment. However,4 mmol/L doxorubicin had remarkable skin cytotoxicity, with only about 40% of cell survival.

|
Download:
|
| Fig. 3.Cytotoxicity evaluation toward skin keratinocytes: (a) untreated control,(b) 4 mmol/L doxorubicin,(c) 1 mL of 300 mmol/L compound 1a dyed sample and (d) 1 mL of 300 mmol/L compound 2 dyed sample. light. | |
|
Download:
|
| Fig. 4.Cell viability of dyed samples containing 1 mL of 300 mmol/L compound 1a and 2 toward skin keratinocytes. Each experiment was conducted in triplicate and a mean value was obtained. Three independent experiments were done. Results are shown as mean ± SD from three independent experiments. light. | |
In summary,the synthesized quinoline compounds were found to be a potentially safe dyestuff for textile coloring treatment. Quinoline compounds (1a and 2)-treated acrylic fabrics showed a satisfactory colourfastness to washing and light. Neither dyed fabrics possessed significant cytotoxicity toward human skin HaCaT cells. The dyed acrylic fabrics could be used for the development of caution apparel due to their good fluorescent effects and low skin cytotoxicity. EN471 Standard is useful in identifying the potential visibility of our dyed acrylic fabrics when the fabrics are applied to caution apparel. In the EN471 Standard, three visibility levels of protection are classified in headlines during darkness. They include Class 1 (the lowest visibility level), Class 2 (the intermediate visibility level),and Class 3 (the highest visibility level). The visibility level of the dyed samples might provide more specifications for caution apparel. Therefore,further work is on-going to investigate the visibility level of the dyed acrylic fabrics by using the EN471 Standard for the potential application in caution apparel. Acknowledgments
This work forms part of the B.A. thesis of S.S.W. Ho. The cellular cytotoxicity study was supported by the starting fund to C.H. Chui (No. 38-40-116). We thank the National Basic Research Program of China (973 Program) (No. 2013CB834702),the 985 Program and 111 Project from Minzu University of China (Nos. CUN985-07-08 and 111 Project B08044),Areas of Excellence Scheme,University Grants Committee of HKSAR,China (No. AoE/P-03/08) and the Science,Technology and Innovation Committee of Shenzhen Municipality (No. JCYJ20120829154440583) for financial support.
| [1] | The World Commission on Environment and Development, From One Earth to One World: An Overview, Oxford University Press, Oxford, 1987, pp. 9-24. |
| [2] | W. Brack, K. Schirmer, Effect-directed identification of oxygen and sulfur heterocycles as major polycyclic aromatic cytochrome P4501A-inducers in a contaminated sediment, Environ. Sci. Technol. 37 (2003) 3062-3070. |
| [3] | S. Meyer, H. Steinhart, Effects of heterocyclic PAHs (N, S, O) on the biodegradation of typical tar oil PAHs in a soil/compost mixture, Chemosphere 40 (2000) 359-367. |
| [4] | F. Nemati, R. Saeedirad, Nano-Fe3O4 encapsulated-silica particles bearing sulfonic acid groups as a magnetically separable catalyst for green and efficient synthesis of functionalized pyrimido[4,5-b]quinolines and indeno fused pyrido[2,3-d]pyrimidines in water, Chin. Chem. Lett. 24 (2013) 370-372. |
| [5] | N. Shahabadi, M. Maghsudi, Gel electrophoresis and DNA interaction studies of the food colorant quinoline yellow, Dyes Pigments 96 (2013) 377-382. |
| [6] | D.C. Mungra, H.G. Kathrotiya, N.K. Ladani, M.P. Patel, R.G. Patel, Molecular iodine catalyzed synthesis of tetrazolo[1,5-a]-quinoline based imidazoles as a new class of antimicrobial and antituberculosis agents, Chin. Chem. Lett. 23 (2012) 1367-1370. |
| [7] | V.B. Kovalska, M.Y. Losytskyy, D.V. Kryvorotenko, et al., Synthesis of novel fluorescent styryl dyes based on the imidazo[1,2-a]pyridinium chromophore and their spectral-fluorescent properties in the presence of nucleic acids and proteins, Dyes Pigments 68 (2006) 39-45. |
| [8] | M. Rahimizade, M. Pordel, M. Bakavoli, H. Eshghi, The synthesis of highly fluorescent heterocyclic compounds: pyrido[20,10:2,3]imidazo[4,5-b] quinoline-12-yl cyanides, Dyes Pigments 86 (2010) 266-270. |
| [9] | Z.X. Han, H.Y. Luo, X.B. Zhang, et al., A ratiometric chemosensor for fluorescent determination of Hg2+ based on a new porphyrin-quinoline dyad, Spectrochim. Acta Mol. Biomol. Spectrosc. 72 (2009) 1084-1088. |
| [10] | R. Stahlmann, H. Lode, Toxicity of quinolones, Drugs 58 (1999) 37-42. |
| [11] | S.H. Chan, C.H. Chui, S.W. Chan, et al., Synthesis of 8-hydroxyquinoline derivatives as novel antitumor agents, ACS Med. Chem. Lett. 4 (2013) 170-174. |
| [12] | P.L. Lam, C.W. Kan, M.C.W. Yuen, et al., Studies on quinoline type dyes and their characterization studies on acrylic fabric, Color. Technol. 128 (2012) 192-198. |
| [13] | AATCC Test Method 61-2003, Colorfastness to Laundering, Home and Commercial: Accelerated, American Association of Textile Chemists and Colorists, AATCC Technical Manual, Research Triangle Park, USA, 2004, pp. 90-94. |
| [14] | AATCC Test Method 16-2003, Colorfastness to Light, American Association of Textile Chemists and Colorists, AATCC Technical Manual, Research Triangle Park, USA, 2006, pp. 23-34. |
| [15] | K.H. Lam, R. Gambari, K.K.H. Lee, et al., Preparation of 8-hydroxyquinoline derivatives as potential antibiotics against Staphylococcus aureus, Bioorg. Med. Chem. Lett. 24 (2014) 367-370. |