Recently,triazole derivatives have attracted considerable attention not only because of their importance as effective and convenient linkages in biological conjugation chemistry [1, 2] and polymer chemistry [3],but also their potentials as pharmacophores in medicinal chemistry [4, 5]. Besides classic 1,4-disubstituted- 1,2,3-triazoles,the modifications on the 5-position of 1,2,3- triazoles proved an alternative effective approach in drug design [6, 7]. Thus simple and effective synthetic methodologies for the derivatization on the 5-position of 1,2,3-triazoles are in great need. Several protocols have been reported to address this issue including the cycloaddition reactions between substituted-alkynes and organic azides [8],electrophilic trapping of triazolyl copper intermediate [9] and C-H activation of the 5-H in 1,2,3-triazoles [10]. But these methods require the synthesis of the unstable 1- iodoalkynes,expensive palladium reagents and complex ligands, or have narrow structural diversity on both reactants and substituents,which limit their further applications in the synthesis of structurally complex compounds and drugs. During our exploration of 1,2,3-triazoles as nucleobase mimics [11],we have developed a series of multi-component reactions to synthesize 5- substituted-1,2,3-triazoles [9b, c, e, 12],providing usful synthetic tools for the triazole-based drug discovery [6, 13]. In this paper,we report our recent progress in synthesizing 5-alkynyl-1,2,3- triazoles through a one-pot aerobic oxidative coupling reaction of various alkynes and azides,and their conversion to other 5- functionalized-1,2,3-triazoles via oxidation,reduction and condensation reactions.
Traditional methods for the preparation of 5-alkynyl-1,2,3- triazoles use a two-step sequence involving the synthesis of 5- iodo-1,2,3-triazole followed by a Sonogashira reaction with terminal alkynes. Porco et al. reported the ligand effects on the oxidative-CuAAC reaction and developed a ligand-controlled synthesis of 5-alkynyl-1,2,3-triazoles using NMO as an oxidant [14]. During our preparation of this paper,Alonso and coworkers also reported their work of constructing 1-benzyl-5-alkynyl-1,2,3- triazoles from benzyl bromide,azide sodium and alkynes [15]. In our work,we found the simple temperature regulation could dramatically improve the yields of 5-alkynyl-1,2,3-triazoles using azides and alkynes as starting materials. Furthermore,this methodology showed a wider scope of substrates such as ribosyl azide,which could not be prepared directly from simple halide. 2. Experimental
The 1H NMR and 13C NMR spectra were recorded at 400 MHz and 100 MHz,respectively. Chemical shifts were reported in ppm from the residual solvent signal or tetramethylsilane (TMS) as an internal standard. Multiplicity was indicated as follows: s (singlet); d (doublet); t (triplet);m (multiplet); dd (doublet of doublets),etc. and coupling constants were given in Hz. The conversion of starting materials was monitored by thin layer chromatography (TLC) analysis using silica gel plates (silica gel 60 F254,0.25 mm) and components were visualized by observation under UV light (254 and 365 nm). Experimental procedures and spectroscopic data are detailed in the supporting information.
Preparation of 5-alkynyl-1,2,3-triazole 3a: Benzyl azide (21 mg, 0.16 mmol),phenylacetylene (40.2 mg,0.4 mmol),CuBr (2.3 mg, 0.016 mmol),and KOH (17.7 mg,0.32 mmol) were added to 1.5 mL of ethanol. The reaction mixture was stirred at 60 ℃ for 10 h and cooled to room temperature. The solvent was evaporated and the residue was partitioned between ethyl acetate and H2O. The organic layer was washed with saturated NH4Cl solution and H2O, then dried over anhydrous Na2SO4 and evaporated. The crude product was purified by silica gel column chromatography (5:1, petroleum ether/ethyl acetate) to give compound 3a as a yellow solid (37.5 mg,71%).
Preparation of 1-(1-benzyl-4-phenyl-1H-1,2,3-triazol-5-yl)-2- phenylethane-1,2-dione 4: A 50 mL flask,immersed in a water bath at 25 ℃,was charged with acetone (10 mL) and 1-benzyl-4- phenyl-5-(phenylethynyl)-1H-1,2,3-triazole (33.5 mg,0.1 mmol). To this mixture was added a solution of NaHCO3 (13.6 mg, 0.162 mmol) and MgSO4 (136 mg,0.552 mol) in water (6 mL). The mixture was stirred with a magnetic stirrer. Powdered potassium permanganate (60 mg,0.39 mmol) was added in one portion,and the mixture was stirred for 8 h. The unreacted permanganate and the precipitated MnO2 were reduced to soluble Mn2+ ions by adding a minimum quantity of NaNO2 (19 mg) and 10% H2SO4 (1 mL). The solution was transferred to a 50 mL separating funnel, saturated with NaCl,and extracted with a hexane-ether mixture. The organic solvents were removed using a rotary evaporator. The residue was dissolved in ether (5 mL) and extracted with a dilute 5% NaOH solution to remove any carboxylic acids present. The ether layer was washed with a saturated solution of NaCl and dried (anhydrous Na2SO4,and the solvent was removed in a rotary evaporator to produce a yellow solid (29.4 mg,80%). 3. Results and discussion
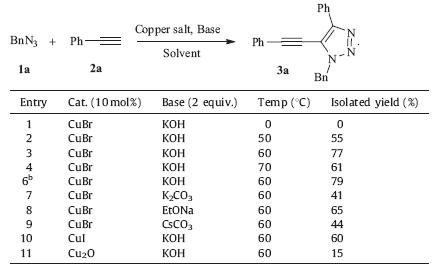
Through the optimization of reaction temperatures,catalysts, solvents and bases (Table 1),60 ℃ was found to be the best reaction temperature,CuBr was the best copper catalyst,and KOH was the best inorganic base. In the presence of 10 mol% CuBr and KOH,phenylacetylene 2a and benzyl azide 1a reacted at 60 ℃ to yield 5-alkynyl-1,2,3-triazole 3a in 77% yield. Regioselectivity of this reaction was confirmed by the X-ray diffraction (XRD) analysis of compound 3a as shown in Fig. S1 in Supporting information, which showed the regioselective alkynyl substitution on the 5- position of 1,4-disubstituted-1,2,3-triazole.
| Table 1 Optimization of reaction conditions for the synthesis of 5-alkynyl-1,2,3-triazole 3a.a |
The different azides and terminal alkynes were applied to investigate the scope of this method (Table 2). Substituted aromatic alkynes with both electron-withdrawing and electrondonating substituents reacted smoothly to produce 5-alkynyl- 1,2,3-triazoles in high yields (68%-71%). For the more bulky 1- ethynylpyrene,a 64% yield of 5-alkynyl-1,2,3-triazole 3e was still obtained. Aliphatic alkyne reacted with 1a to produce compounds 3f and 3g in 75% and 65% yields,respectively. Phenethyl azide as the azide donor reacted smoothly with a terminal alkyne to produce 5-alkynyl-1,2,3-triazole 3h in 75% yield. Unnatural nucleosides are valuable biological tools and bioactive molecules [16]. Connection of ribosyl azide and designed alkynyl building blocks by CuAAC reaction provided an effective approach to this kind of compounds [4b]. Herein we found that ribosyl azide donor was also an effective substrate for the preparation of 5-alkynyl- 1,2,3-triazolyl nucleoside 3i in 67% yield under standard conditions.
| Table 2 Synthesis of 5-alkynyl-1,2,3-triazoles 3.a |
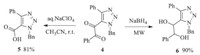
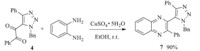
With 5-alkynyl-1,2,3-triazoles in hand,we explored next the potential of 5-alkynyl-1,2,3-triazoles as precursors to other 5- functionalized-1,2,3-triazoles (Schemes 1-3). It was difficult to introduce an electron-withdrawing group on the 5-position of 1,2,3-triaozle by the CuAAC reactions. Here we could successfully transform 5-alkynyl-1,2,3-triazole 3a to 5-dicarbonly-1,2,3-triazole 4 via an oxidative reaction in presence of KMnO4 (Scheme 1). And then,the 5-hydroxyalkyl-1,2,3-triazole 5,5-carboxyl-1,2,3- triazoles 6 could be prepared from 4 in excellent yields,by an oxidation and a reduction reaction,respectively (Scheme 2). Quinoxaline is a classic pharmacophore in current drug design. 5- Quinoxaline-1,2,3-triazole 7 could also be effectively prepared by a condensation reaction between 5-dicarbonly-1,2,3-triazole 4 and benzene-1,2-diamine (Scheme 3).

|
Download:
|
| Scheme 1.Synthesis of 5-dicarbonly-1,2,3-triazole 4. | |
|
Download:
|
| Scheme 2.Synthesis of 5-hydroxyalkyl-1,2,3-triazole 5 and 5-carboxyl-1,2,3- triazoles 6. | |
|
Download:
|
| Scheme 3.Synthesis of 5-quinoxaline-1,2,3-triazole 7. | |
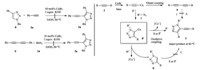
For the mechanism of the formation of 5-alkynal-1,2,3-triazoles from alkynes and azides,three possible routes (A,B,and C) showed in Scheme 4 based on the mechanisms of the Cu(I) mediated click reaction and Glaser reaction of alkynes in the literatures [8a, 17] can be envisioned. Two additional experiments were also performed (Scheme 4): under the current reaction conditions, compound 8 cannot react with 2a to form 5-alkynyl-1,2,3-triazole 3a,and Glaser coupling product 1,4-diphenyl-1,3-diyne 9 cannot either react with azide 1a to form 3a,which rule out the possibilities of forming 3 via 8 as an intermediate (Mechanism C) or via Cu(I) mediated Glaser coupling of an alkyne to a 1,3-diyne followed by the click reaction with azide 2 (Mechanism A). These results revealed that the CuAAC reaction should precede the oxidative coupling reaction in the sequence. Thus it seems that the reaction proceeds through the aerobic coupling of the carbanion intermediate 8' of the CuAAC reaction with the copper acetylide 2' to afford the corresponding 3 (mechanism B). In this procedure,the temperature might play a role to regulate reaction pathways. At 60 ℃,aerobic oxidative coupling reaction preferentially takes place between intermediate 8' and copper acetylide 2' to produce 5-alkynly-1,2,3-triaozle 3 chemoselectively.
|
Download:
|
| Scheme 4.The possible reaction mechanism. | |
In conclusion,a simple,efficient and ligand-free synthesis of 5- alkynyl-1,2,3-triazoles has been developed using a one-pot aerobic oxidative CuAAC reactions at 60 ℃. Various azides and alkynes can be used as the substrates in these reactions to achieve chemical diversity. In particular,the successful application of the CuBr/Base catalytic system in sugar indicates the value of this method in the synthesis of designed unnatural nucleoside. Furthermore,the transformation of 5-alkynyl-1,2,3-triazoles to other structurally diverse 5-functionalized-1,2,3-triazoles illustrates the potential of our methods in the triazole-based drug design. Acknowledgments
We are grateful to the NSFC (Nos. 21172058,20802017), HASTIT (No. 2012HASTIT10),PCSIRT (No. IRT1061) and Key Technologies R & D Program of Henan Province (No. 112102310319) for financial support. Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.03.004.
| [1] | A.H. El-Sagheer, T. Brown, Click chemistry with DNA, Chem. Soc. Rev. 39 (2010) 1388-1405. |
| [2] | (a) M. van Dijk, D.T.S. Rijkers, R.M.J. Liskamp, C.F. van Nostrum, W.E. Hennink, Synthesis and applications of biomedical and pharmaceutical polymers via click chemistry methodologies, Bioconjugate Chem. 20 (2009) 2001-2016; (b) V. Hong, S.I. Presolski, C. Ma, M.G. Finn, Increasing the efficacy of bioorthogonal click reactions for bioconjugation: a comparative study, Angew. Chem. Int. Ed. 48 (2009) 9879-9883. |
| [3] | (a) J. Lutz, 3-Dipolar cycloadditions of azides and alkynes: a universal ligation tool in polymer and materials science, Angew. Chem. Int. Ed. 46 (2007) 1018-1025; (b) P.L. Golas, K. Matyjaszewski, Marrying click chemistry with polymerization: expanding the scope of polymeric materials, Chem. Soc. Rev. 39 (2010) 1338-1354. |
| [4] | (a) G.C. Tron, T. Pirali, R.A. Billington, et al., Click chemistry reactions in medicinal chemistry: applications of the 1,3-dipolar cycloaddition between azides and alkynes, Med. Res. Rev. 28 (2008) 278-308; (b) F. Amblard, J.H. Hyun Cho, R.F. Schinazi, Cu(Ⅰ)-catalyzed huisgen azide-alkyne 1,3-dipolar cycloaddition reaction in nucleoside, nucleotide, and oligonucleotide chemistry, Chem. Rev. 109 (2009) 4207-4220. |
| [5] | S.G. Agalave, S.R. Maujan, V.S. Pore, Click chemistry: 1,2,3-triazoles as pharmacophores, Chem. Asian J. 6 (2011) 2696-2718. |
| [6] | R. De Simone, M.G. Chini, I. Bruno, et al., Structure-based discovery of inhibitors of microsomal prostaglandin E2 synthase-1,5-lipoxygenase and 5-lipoxygenaseactivating protein: promising hits for the development of new anti-inflammatory agents, J. Med. Chem. 54 (2011) 1565-1575. |
| [7] | D. Imperio, T. Pirali, U. Galli, et al., Replacement of the lactone moiety on podophyllotoxin and steganacin analogues with a 1,5-disubstituted 1,2,3-triazole via ruthenium-catalyzed click chemistry, Bioorg. Med. Chem. 15 (2007) 6748-6757. |
| [8] | (a) J.E. Hein, J.C. Tripp, L.B. Krasnova, K.B. Sharpless, V.V. Fokin, Copper(Ⅰ)-catalyzed cycloaddition of organic azides and 1-iodoalkynes, Angew. Chem. Int. Ed. 48 (2009) 8018-8021; (b) B.H.M. Kuijpers, G.C.T. Dijkmans, S. Groothuys, et al., Copper(Ⅰ)-mediated synthesis of trisubstituted 1,2,3-triazoles, Synlett (2005) 3059-3062; (c) J.H. Huang, S.J.F. Macdonald, J.P.A. Harrity, A cycloaddition route to novel triazole boronic esters, Chem. Commun. (2009) 436-438. |
| [9] | (a) Y.M. Wu, J. Deng, Y. Li, Q.Y. Chen, Regiospecific synthesis of 1,4,5-trisubstituted-1,2,3-triazole via one-pot reaction promoted by copper(Ⅰ) salt, Synthesis (2005) 1314-1318; (b) L. Li, G. Zhang, A. Zhu, L. Zhang, A convenient preparation of 5-iodo-1,4-disubstituted-1,2,3-triazole: multicomponent one-pot reaction of azide and alkyne mediated by CuI-NBS, J. Org. Chem. 130 (2008) 3630-3633; (c) L. Li, R. Li, A. Zhu, G. Zhang, L. Zhang, CuBr-NCS-mediated azide-alkyne cycloaddition: mild and effient synthesis of 5-bromo-1,4-disubstituted-1,2,3-triazoles, Synlett (2011) 874-878; (d) R. Yan, K. Sander, E. Galante, et al., A one-pot three-component radiochemical reaction for rapid assembly of 125I-labeled molecular probes, J. Am. Chem. Soc. 135 (2013) 703-709; (e) L. Li, G. Hao, A. Zhu, S. Liu, G. Zhang, Three-component assembly of 5-halo-1,2,3-triazoles via aerobic oxidative halogenation, Tetrahedron Lett. 54 (2013) 6057-6060. |
| [10] | (a) S. Chuprakov, N. Chernyak, A.S. Dudnik, V. Gevorgyan, Direct Pd-catalyzed arylation of 1,2,3-triazoles, Org. Lett. 9 (2007) 2333-2336; (b) L. Ackermann, R. Jeyachandran, H.K. Potukuchi, P. Novak, L. Buettner, Palladium-catalyzed dehydrogenative direct arylations of 1,2,3-triazoles, Org. Lett. 12 (2010) 2056-2059; (c) H. Jiang, Z. Feng, A. Wang, X. Liu, Z. Chen, Palladium-catalyzed alkenylation of 1,2,3-trizoles with terminal conjugated alkenes by direct C-H bond functionalization, Eur. J. Org. Chem. (2010) 1227-1230; (d) L. Ackermann, R. Vicente, R. Born, Palladium-catalyzed direct arylations of 1,2,3-triazoles with aryl chlorides using conventional heating, Adv. Synth. Catal. 350 (2008) 741-748. |
| [11] | L. Li, C.C. Siebrands, Z. Yang, et al., Novel nucleobase-simplified cyclic ADP-ribose analogue: a concise synthesis and Ca2+-mobilizing activity in T-lymphocytes, Org. Biol. Chem. 50 (2010) 1843-1848. |
| [12] | L. Li, G. Hao, A. Zhu, et al., A copper(Ⅰ)-catalyzed three-component domino process: assembly of complex 1,2,3-triazolyl-5-phosphonates from azides, alkynes, and hphosphates, Chem. Eur. J. 19 (2013) 14403-14406. |
| [13] | J.C. Morris, J. Chiche, C. Grellier, et al., Targeting hypoxic tumor cell viability with carbohydrate-based carbonic anhydrase IX and XII inhibitors, J. Med. Chem. 54 (2011) 6905-6918. |
| [14] | B. Gerard, J. Ryan, A.B. Beeler, J.A. Porco Jr., Synthesis of 1,4,5-trisubstituted-1,2,3-triazoles by copper-catalyzed cycloaddition-coupling of azides and terminal alkynes, Tetrahedron 62 (2006) 6405-6411. |
| [15] | F. Alonso, Y. Moglie, G. Radivoy, M. Yusa, lCettoerpper-catalysed multicomponent click synthesis of 5-alkynyl 1,2,3-triazoles under ambient conditions, Synlett 23 (2012) 2179-2182. |
| [16] | L.J. Li, M. Degardin, T. Lavergne, et al., Natural-like replication of an unnatural base pair for the expansion of the genetic alphabet and biotechnology applications, J. Am. Chem. Soc. 136 (2014) 826-829. |
| [17] | L.J. Li, J. Wang, G. Zhang, Q. Li, A mild copper-mediated Glaser-type coupling reaction under the novel CuI/NBS/DIPEA promoting system, Tetrahedron Lett. 50 (2009) 4033-4036. |