The safety of a drug product is not only dependent on the toxicological properties of the active drug substance (or API),but also on the impact of impurities formed during the various chemical transformations. Therefore,the development of a drug substance,especially for a chiral drug,is incomplete without the identification and characterization of its stereoisomer profile [1- 3]. Strict regulatory guidelines of the International Conference on Harmonization (ICH) have led to an increasing need for identification, quantification and control of trace impurities in the drug substances and drug products to obtain marketing approval [4, 5, 6]. However,it is more challenging to identify the impurities that are formed in very small quantities. Since frequently it is very difficult to identify and control impurities within acceptable levels in the process,extra purification steps may then be necessary thereby making the process less competitive. More often than not,the syntheses of impurities are not described in the literature,which posts a greater challenge [1].
Ezetimibe 1 shown in Fig. 1 has been commercialized as an effective inhibitor of intestinal cholesterol and related phytosterol absorption for lowering cholesterol levels [7, 8, 9]. As three asymmetric carbons in the Ezetimibe molecule give rise to eight stereoisomers,the synthesis of the final products with the required stereochemistry is a significant challenge [10]. However,stereoisomers of chiral drugs often show different behaviors in pharmacological action and metabolic process. It is definitely common that one stereoisomer is active while other stereoisomers are toxic in biological systems. The pharmaceutical industry has recently raised its emphasis on the generation of single optical isomer before undertaking pharmacokinetic,metabolic,physiological, and toxicological evaluation in the search for drugs with greater therapeutic benefits and lower toxicity [11]. During the laboratory optimization and the late phase manufacturing studies of Ezetimibe 1,low levels (<0.1%) of SRREzetimibe 2 and RRS-Ezetimibe 4 were discovered as shown in Fig. 2. In our previous research [12],we have successfully designed an unambiguous synthetic approach to prepare SRR-Ezetimibe 2 and its enantiomer RSS-Ezetimibe 3. Single-crystal X-ray analysis of SRR-Ezetimibe 2 [13] provided the final verification of the structural assignments. Considering that the fact that the synthesis and characterization of stereoisomers 4-8 is almost completely overlooked,we explored the synthesis and characterization of stereoisomers 4-8 of Ezetimibe 1 for the first time in this study. It is essential to characterize the complete stereoisomer profile of Ezetimibe 1 and meet the stringent regulatory requirements of ICH. These results will be of immense help for organic chemists to understand the potential stereoisomers in Ezetimibe 1 API and may have some implications in the development of new medicines [12].

|
Download:
|
| Fig. 1.Ezetimibe 1,process related stereoisomers 2-8. | |

|
Download:
|
| Fig. 2.HPLC chromatogram of the crude Ezetimibe 1. | |
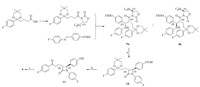
stereoisomers 4-8 are major byproducts in the preparation of the crude Ezetimibe 1 API,and difficult to be purified by column chromatography. Herein,we design an efficient route to synthesize the stereoisomers 4-8. As shown in Scheme 1,compound 9a on reaction with N,O-bis(trimethylsilyl)acetamide and tetrabutylammonium fluoride trihydrate in acetonitrile at 25°C gave the cyclized b-lactam product 10. b-Lactam product 10 was treated with 2 mol/L H2SO4 in i-PrOH to get the ketone 11 which is purified by column chromatography. S-Methyloxazaborolidine-mediated reduction of ketone 11 with borane dimethyl-sulfide at -5°C delivered the pure RRS-Ezetimibe 4 after recrystallization from i-PrOH and water.
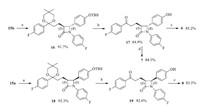
The same strategy to synthesize the stereoisomer 4 can be used to prepare stereoisomer 5 when we employed compound 9b as our starting material as shown in Scheme 2.
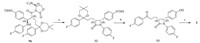
As mentioned in Scheme 3,our synthesis approach to prepare stereoisomer 6-8 firstly began with the coupling of 4-(2-(4- fluorophenyl)-5,5-dimethyl-1,3-dioxan-2-yl)butanoic acid with (R)-4-phenyl-2-oxazolidinone which was done by the mixed anhydride method using pivaloyl chloride and triethylamine to obtain oxazolidinone 14. The crucial enolate condensation of oxazolidinone 14 with N-(4-((tert-butyldimethylsilyl)oxy)benzylidene)- 4-fluoroaniline in the presence of TiCl4 and Ti(OiPr)4 at -40°C delivered the key intermediates 15a and 15b with the ratio of two stereoisomers (15a:15b) about 1:8.
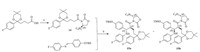
Based on the mechanism of Ti(IV)-catalyzed Mannich-type equilibrium reaction and the fact that all the physical properties (melting point,ESI-MS,NMR) of the intermediates 15a and 9b are identical except for the optical rotation values,which are of opposite sign,it can be concluded that the intermediates 15a and 9b are a pair of enantiomers. Same is true for the intermediates 15b and 9a. Having established the absolute configuration of intermediates 15a and 15b,we were able to obtain stereoisomer 6-8 using a similar sequence as shown in Scheme 4.
|
Download:
|
| Scheme 1.Reagents and conditions: (a) BSA,TBAF·3H2O,CH3CN,25°C,86.7%; (b) 2 mol/L H2SO4,i-PrOH,50°C,79.6%; (c) S-CBS,94% BH3-DMS,DCM,-5°C,82.3%. | |
|
Download:
|
| Scheme 2.Reagents and conditions: (a) BSA,TBAF·3H2O,CH3CN,25°C,92.8; (b) 2 mol/L H2SO4,i-PrOH,50°C,80.3%; (c) S-CBS,94% BH3-DMS,DCM,-5°C,84.9%. | |
|
Download:
|
| Scheme 3.Reagents and conditions: (a) pivaloyl chloride,TEA,DCM,r.t.; (R)-4-phenyloxazolidin-2-one,LiCl,45 8C,78.5%; (b) TiCl4,Ti(OiPr)4,DIEA,DCM,-40 8C. | |
|
Download:
|
| Scheme 4.Reagents and conditions: (a) BSA,TBAF·3H2O,CH3CN,25°C; (b) 2 mol/L H2SO4,i-PrOH,50°C; (c) R-CBS,94%BH3-DMS,DCM,-5°C; (d) S-CBS,94% BH3-DMS,DCM,0 8C. | |
Based on a comprehensive review of the literature,we found little research on the synthesis of the stereoisomers 4-8 related to Ezetimibe,which represents a defect and deficiency in the quality analysis and quality control of Ezetimibe API. Katarzyna Filip and his co-workers [10] indicated that the formation of RRS-Ezetimibe 4 is inconvenient because this impurity is difficult to remove from the final product Ezetimibe 1. Compound EZ-6,which is a key intermediate to prepare RRR-Ezetimibe 5 as shown in Scheme 5, was not isolated in Filip’s work. We also failed to observe the desired RRS-Ezetimibe 4 and RRR-Ezetimibe 5 under the reported reaction conditions or their slight variations. Gratifyingly,the disappointing results have prompted us to identify an alternative and practical method to access stereoisomers 4-8 of Ezetimibe 1 as shown in Schemes 1-4.
The RRS-Ezetimibe 4 was a main by-product when we used Rmethyloxazaborolidine- mediated reduction of ketone 11 with borane dimethyl-sulfide to prepare the crude Ezetimibe 1 API [14- 16]. The polarity of RRS-Ezetimibe 4 and Ezetimibe 1 is so close that they are difficult to be separated completely even using HPLC as shown in Fig. 2. High levels (>3%) of RRS-Ezetimibe 4 may be monitored by HPLC when the temperature was unsatisfactory. The RRS-Ezetimibe 4 with high purity can be prepared and recrystallized from i-PrOH and water when we employed S-methyloxazaborolidine to replace the R-methyloxazaborolidine as shown in Scheme 1. Thus,we can make full use of the RRS-Ezetimibe 4 to establish an HPLC method for the analysis of Ezetimibe 1 API.
In our previous work [12],we have confirmed the absolute configuration of intermediate 9b using single-crystal X-ray analysis of the final product SRR-Ezetimibe 2 derived from 9b. In our large scale preparation of Ezetimibe 1 API,we can recrystallize the pure compound 9b from the concentrated mother liquor after removing the main product 9a. Thus,the same strategy to synthesize the stereoisomer 2 can be used to prepare stereoisomer 5 when we employed S-methyloxazaborolidine to replace the R-methyloxazaborolidine in the reduction of ketone 13 with borane dimethyl-sulfide as shown in Scheme 2.
The impressive stereodirecting power of (S)-4-phenyl-2-oxazolidinone in the crucial enolate condensation suggests that its enantiomer (R)-4-phenyl-2-oxazolidinone can be used to generate the key chiral intermediates 15a and 15b as shown in Scheme 3 [12]. In some of commercial samples,(S)-4-phenyl-2-oxazolidinone was found to be contaminated with trace amount of (R)-4- phenyl-2-oxazolidinone,which is why low levels (<1%) of intermediates 15a and 15b may be detected in the key intermediate 9a when we prepare the Ezetimibe 1 API. This is also the reason why trace amount of stereoisomer 6-8 may be detected in the crude Ezetimibe 1.

|
Download:
|
| Scheme 5.The route to prepare RRR-Ezetimibe 5 of Katarzyna Filip and his co-workers. | |
In summary,to better understand the synthetic pathway of an active pharmaceutical ingredient (API),we have synthesized and completely characterized several key stereoisomers of Ezetimibe 1 for the first time. This research,together with previous work [12], completed the synthesis and characterization of all stereoisomers related to Ezetimibe 1,which is essential to establish the complete stereoisomer profile of Ezetimibe 1 to meet the stringent regulatory requirements of ICH. Our research will provide an access to the reference standard of these stereoisomers and may have some implications in the development of new medicines. Acknowledgments
We would like to express our gratitude to Sichuan Provincial Science and Technology Support Program (No. 2011SZ0014) and Sichuan Bali Pharmaceutical Co.,Ltd. for financial support.
| [1] | I.V.S. Kumar, G.S.R. Anjaneyulu, V.H. Bindu, Identification and synthesis of impurities formed during sertindole preparation, Beilstein J. Org. Chem. 7 (2011) 29-33. |
| [2] | C.S. Wu, Z.X. Jia, B.M. Ning, J.L. Zhang, S. Wu, Separation and identification of moxifloxacin impurities in drug substance by high-performance liquid chromatography coupled with ultraviolet detection and Fourier transform ion cyclotron resonance mass spectrometry, Chin. Chem. Lett. 23 (2012) 1185-1188. |
| [3] | W.L. Wan, Y. He, M. Guan, et al., Synthesis of the major isomers of Aprepitant and Fosaprepitant, Chin. Chem. Lett. 24 (2013) 1118-1122. |
| [4] | International Conference on Harmonization (ICH) Guidelines, Q3A (R) Impurities in New Drug Substances, 2002 (this guideline provides guidance for registration application on the content and qualification of impurities in new drug substances produced by the chemical synthesis). |
| [5] | International Conference on Harmonization (ICH) guidelines, Q3B (R) Impurities in New Drug Substances, 2002 (Guidance for registration or marketing application on the content and qualification of impurities in new drug product). |
| [6] | M. Vuletić, M. Cindrić, J.D. Koružnjak, Identification of unknown impurities in simvastatin substance and tablets by liquid chromatography/tandem mass spectrometry, J. Pharm. Biomed. Anal. 37 (2005) 715-721. |
| [7] | H. Bays, Ezetimibe, Expert Opin. Investig. Drugs 11 (2002) 1587-1604. |
| [8] | S.D. Turley, J.M. Dietschy, Sterol absorption by the small intestine, Curr. Opin. Lipidol. 14 (2003) 233-240. |
| [9] | H.R. Davis, D.S. Compton, L. Hoos, G. Tetzloff, Ezetimibe, a potent cholesterol absorption inhibitor, inhibits the development of atherosclerosis in apoe knockout mice, arterioscler, Thromb. Vasc. Biol. 21 (2001) 2032-2038. |
| [10] | K. Filip, K. Bankowski, K. Sidoryk, et al., Physicochemical characterization of ezetimibe and its impurities, J. Mol. Struct. 991 (2011) 162-170. |
| [11] | K. Zhang, N. Xue, Z.F. Yuan, et al., Separation of the two enantiomers of naproxcinod by chiral normal-phase liquid chromatography, J. Chromatogr. Sci. 49 (2011) 272-275. |
| [12] | Y. Ren, R.J. Li, Y. Deng, et al., First synthesis and characterization of SRR/RSSEzetimibe, Tetrahedron Lett. 54 (2013) 6443-6446. |
| [13] | Crystallographic data (excluding structure factors) for the structures in this paper have been deposited with the Cambridge Crystallographic Data Centre as supplementary publication nos. CCDC 944997. Copies of the data can be obtained, free of charge, on application to CCDC, 12 Union Road, Cambridge CB2 1EZ, UK (fax: +44 01223 336033 or email:deposit@ccdc.cam.ac.uk). |
| [14] | Y. Kawanami, S. Murao, T. Ohga, et al., Practical enantioselective reduction of ketones using oxazaborolidine catalyst generated in situ from chiral lactam alcohol and borane, Tetrahedron 59 (2003) 8411-8420. |
| [15] | G.J. Quallich, T.M. Woodall, Enantioselective oxazaborolidine reduction of ketones containing heteroatoms, Tetrahedron Lett. 34 (1993) 785-788. |
| [16] | J.F. Ji, H.B. Zhang, W.L. Huang, et al., A convenient synthesis of ezetimibe analogs as cholesterol absorption inhibitors, Chin. Chem. Lett. 21 (2010) 67-69. |




