b Department of Chemistry, Teachers College for Nationalities, Qinghai Normal University, Xining 810008, China
The genus Artemisia has been placed taxonomically in the tribe Anthemideae of the family Asteraceae,with 186 species distributed within mainland of China [1]. Artemisia gmelinii Web. ex Stechm.,a perennial herb belonging to the genus Artemisia,is widely distributed in east and south Asia with several ethnopharmacological applications,for the treatment of skin diseases,inflammatory liver conditions,cold,cough,fever,boils,and pimples [2, 3]. A recent pharmacological study has demonstrated the antioxidant activity of methanol extracts of A. gmelinii [2]. Sesquiterpene lactones, flavonoids,coumarins,and organic acid derivatives have been reported to be isolated from the plant [2, 4]. Our current research on the constituents of A. gmelinii Web. ex Stechm. resulted in the isolation of two new oplopane-type sesquiterpenes,gmelinin A (1) and gmelinin B (2) (Fig. 1). This is the first report on the isolation of oplopane sesquiterpenes from the genus Artemisia. 2. Experimental
2.1. General experimental procedures
HR-ESI-MS was recorded on a Waters Xevo G2 Q-TOF mass spectrometer (Waters,Milford,MA,USA). 1D and 2D NMR spectra were taken on a Bruker AV 400 spectrometer (Bruker,Fllanden,Switzerland) with tetramethylsilane (TMS) as the internal standard. High-performance liquid chromatography (HPLC) analysis was performed on an analytical HPLC system (Shimadzu,Kyoto,Japan) consisting of a LC-10AVP pump,a DGU-14A degasser,a SCL-10AVP system controller,a SPDM10AVP diode-array detector,and a Cosmosil ODS column (4.6mm × 250 mm) with a flow rate of 1mL/min. HPLC preparation was performed on a preparative HPLC system (Waters,Milford,MA,USA) equipped with a Waters 600 pump,a Waters 600 controller,a Waters 486 tunable absorbance detector,and a Grace ODS column (Allsphere ODS-2, 10mm×250mm) with a flow rate of 10 mL/min. Column chromatography (CC) was performed on silica gel (200-300 mesh, Qingdao Marine Chemical Co.,Ltd.,Qingdao,China),MDS-5 reversed phase silica gel (Medicine Technology Center,Beijing,China), Sephadex LH-20 (GE Healthcare,Uppsala,Sweden),and SciBioChem MCI GEL (SBC MCI GEL,Chengdu Kepubio Co.,Ltd.,Chengdu,China). Thin layer chromatography (TLC) was performed on silica gel plates (TLC: GF254; Qingdao Marine Chemical Co.,Ltd.,Qingdao,China). Solvents of analytical grade were purchased from Beijing Chemical Corporation (Beijing,China). Solvents of chromatographic gradewere purchased from Xilong Chemical Corporation (Shantou,Guangdong). (R)-MTPA-Cl,(S)-MTPA-Cl and 4-DMAP were purchased from Alfa (Tianjing) Chemical Corporation (Tianjing,China). Fractions were monitored by TLC,and spots were visualized on precoated silica gel plates by spraying 1% vanillin in H2SO4 followed by heating. 2.2. Plant material
The whole plants of A. gmelinii Web. ex Stechm. were collected in Yushu,Qinghai Province,China,in September 2010. Species identification was confirmed by Prof. Skarma Tsogs Gnyis,Tibetan Medical College,Qinghai University,Xining,China. A voucher specimen (FL2010101701) is maintained in the Department of Natural Medicines,School of Pharmaceutical Sciences,Peking University.

|
Download:
|
| Fig. 1.Structures of compounds 1 and 2. | |
The air-dried and powdered whole plants of A. gmelinii (13 kg) were percolated with 95% aqueous EtOH and 50% aqueous EtOH exhaustively at room temperature,respectively. After evaporation of the solvent under reduced pressure,the residues were mixed and suspended in water and then successively partitioned with petroleum ether,EtOAc,and n-BuOH to afford the corresponding extracts. The EtOAc extract (500.0 g) was subjected to silica gel CC (30 cm × 60 cm) and eluted with CHCl3-MeOH (10:0-0:10,v/v) to give seven fractions (Fr.1-Fr.7). Fr.3 (38.0 g) was further separated on silica gel CC (30 cm × 60 cm) and eluted with petroleum ether- acetone (3:1-1:2,v/v) to give five subfractions (fr.3A-fr.3E). 1 (70 mg) was obtained from fr.3C (7.2 g) by MDS-5 reversed phase CC (MeOH-H2O 40:60-100:0,v/v) and Sephadex LH-20 CC (MeOH-H2O 80:20,v/v). Further purification of fr.3D (4.2 g) by MCI GEL CC (MeOH-H2O 40:60-100:0,v/v),preparative HPLC (MeOH-H2O 50:50,flow rate 2.5 mL/min,wavelength 210 nm), and then Sephadex LH-20 CC (MeOH-H2O 80:20,v/v) afforded 2 (10 mg).
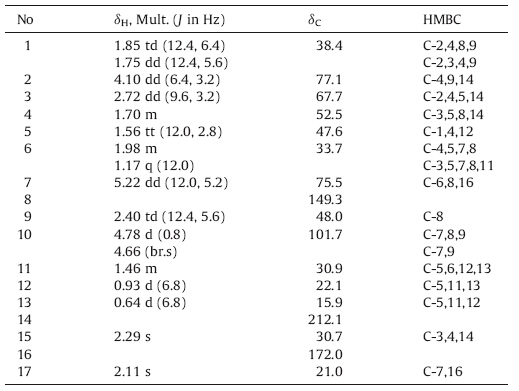
(2R,3R,4S,5S,7S,9R)-2,7-Dihydroxy-8(10) - oplopen-14-one (gmelinin A,1): Colorless needle crystals (MeOH); UV (MeOH) lmax(logε): 212 (3.75) nm; for 1H NMR and 13C NMR spectral data see Table 1; HR-ESI-MS (neg.) m/z: 251.1644 [M_H]- (calcd. for C15H23O3, 251.1647). (2R,3R,4S,5S,7S,9R)-2-Hydroxyl-7-acetoxy-8(10) - oplopen-14- one (gmelinin B,2): Colorless needle crystals (MeOH); UV (MeOH) lmax(logε): 211 (4.01) nm; for 1H NMR and 13C NMR spectral data see Table 3; HR-ESI-MS (neg.) m/z: 293.1761 [M_H]- (calcd. for C17H25O4,293.1753).
| Table 1 1H (400 MHz) and 13C (100MHz) NMR spectral data of 1 in CD3OD. |
Compound 1 (1.0 mg) was dissolved in 500 mL of dry pyridine-d5 in a nuclear magnetic tube,treated with an excess of DMAP (4 mg) and (-)-(R)-MTPA chloride (12 μL),and then placed at room temperature over 12 h to yield the (S)-MTPA diester of 1. Using (+)-(S)-MTPA chloride,the same procedure afforded (R)- MTPA diester. Then 1H NMR and 1H-1H COSY spectra of the mixtures in two nuclear magnetic tubes were taken,respectively. Analysis of the 1H-1HCOSY data led to the correct assignment of the high field 1H NMR signals due to the sesquitepene moiety of the MTPA ester derivatives of compound 1. 2.5. X-ray crystallographic analysis of compound 1
The intensity data collection and structural analysis were performed on a SuperNova,Dual,Cu at zero,Atlas diffractometer. The crystal was kept at 180.00(10) K during data collection. Using Olex2,the structure was solved with the Superflip structure solution program using charge flipping and refined with the XL refinement package using least squares minimization. Crystallographic data have been deposited at the Cambridge Crystallographic Data Centre under the reference number CCDC 988673. Copies are available free of charge on application to the Director, CCDC,12 Union Rd.,Cambridge CB2 1EZ,UK (e-mail: deposit@ ccdc.cam.ac.uk).
Crystal data for compound 1 (M = 252.34): Colorless needle crystals (from MeOH),monoclinic,space group P21 (no. 4), a = 8.9118(4)A˚ ,b = 14.0235(12)A˚ ,c = 17.1647(11)A˚ , b = 90.285(5)8,V = 2145.1(2)A˚ 3,Z = 6,T = 180.00(10) K,m(Mo Ka) = 0.080 mm-1,Dcalc = 1.172 g/mm3,7743 reflections measured (5.904 ≤ 2Θ ≤ 50.044),5840 unique (Rint = 0.0299) which were used in all calculations. The final R1 was 0.0511 (I > 2s(I)) and wR2 was 0.1084 (all data). 3. Results and discussion
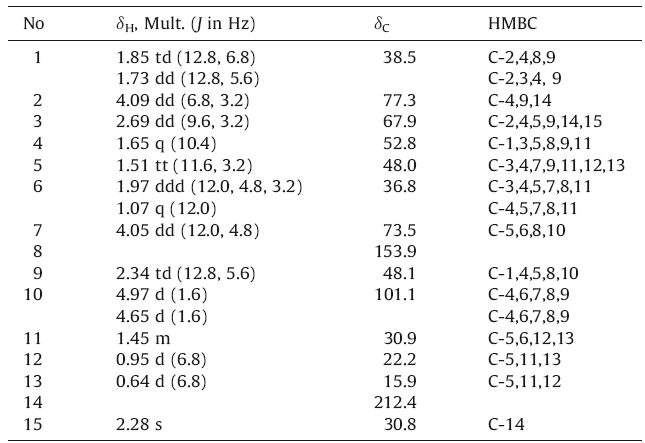
compound 1 was isolated as colorless crystals (MeOH). Its molecular formula was determined to be C15H24O3 by negative HRESIMS (m/z 251.1644 [M-H]-,calcd. for 251.1647),requiring four degrees of unsaturation. The 1H NMR spectrum showed three methyl proton signals at δH 0.95 (d,3H,J = 6.8 Hz),0.65 (d,3H, J = 6.8 Hz) and 2.28 (s,3H),two oxygenated methine proton signals at δH 4.09 (dd,1H,J = 6.8,3.2 Hz) and 4.05 (dd,1H,J = 12.0,4.8 Hz), and two exomethylene proton signals at δH 4.97 (d,1H,J = 1.6 Hz) and 4.65 (d,1H,J = 1.6 Hz). The 13C NMR and DEPT spectra displayed 15 carbon signals including three methyl carbons,three methylene carbons,seven methine carbons,and two quaternary carbons,of which a ketone signal at δC 212.4 and an alkenyl signal at δ C 153.9 were characterized. All the 1H NMR and 13C NMR spectral data of 1 were completely assigned based on 1H-1H COSY, HSQC,and HMBC analyses (Table 1 and Fig. 2).

|
Download:
|
| Fig. 2.Key COSY and HMBC correlations of compounds 1 and 2. | |
|
Download:
|
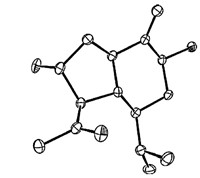
| Fig. 3.X-ray crystal structure of compound 1. | |
Based on the 1H-1H COSY spectrum,two partial fragments for the skeleton of compound 1,CH(7)-CH2(6)-CH(5)-CH(4)-CH(3)- CH(2)-CH2(1)-CH(9) and CH3(13)-CH(11)-CH3(12) were established, and a five membered ring of CH2(1)-CH(2)-CH(3)-CH(4)- CH(9) was deduced from the 1H-1H COSY correlation of H-4 and H- 9. HMBC correlations between the olefinic protons H2-10 (δH 4.97, 4.65) and C-7 (δC 73.5),C-9 (δC 48.1),C-8 (δC 153.9) established the presence of an 8-exomethylene cyclohexane substructure,which was fused to the five membered ring through C-4 and C-9. The C-C interconnectivity of the two fragments was deduced fromthe HMBC correlations from H-11 to C-5 and C-6. HMBC correlations of H3-15 at δ 2.28 with C-3 at δ 67.9 and C-14 at δ 212.4 and H-2 at δ 4.09 with C-14 at δ 212.4 indicated the position of the acetyl group. Evidenced by the above information,the planar structure of 1 was fully constructed to be an oplopanol derivative as shown in Fig. 1 [5].
The relative configuration of 1 was assessed by detailed inspection of its NOESY spectrum and the X-ray crystallography results. Obvious NOESY correlations of H-7 with H-5 and H-9,H-3 with H-5 indicated that H-3,5,7,9 were all oriented on the same face of the molecule,while H-4 and H-2 were deduced to be on the other face based on the NOESY correlation of H-4 with H-2. The deduced relative configuration of 1 was confirmed unequivocally by X-ray crystallography (see Fig. 3). Subsequently,the modified Mosher method was used for the determination of its absolute configuration. Treatment of two aliquots of 1 with (-)-(R) and (+)- (S) MTPA chloride in dry pyridine-d5 in a nuclear magnetic tube gave the corresponding diesters 1S and 1R,respectively. The pattern of Δδ (S-R) values (see Table 2 and Fig. 4) resulted in 2R and 7S assignment and 3S,4R,5S and 9S were assigned accordingly.Thus 1,was established as (2R,3R,4S,5S,7S,9R)-2,7-dihydroxy- 8(10)-oplopen-14-one which was a new compound and given the name gmelinin A.

|
Download:
|
| Fig. 4.Application of the modified Mosher’s method to determine the absolute configuration at C-2 and C-7 of compound 1. Δδ (S-R) values are given in ppm. | |
| Table 2 The high field signals of 1H (400MHz) NMR spectral data of 1R and 1S in pyridine-d5. |
| Table 3 1H (400MHz) and 13C (100MHz) NMR spectral data of 2 in CD3OD. |
Currently,54 oplopane sesquiterpenes have been isolated from nature resources,most of which are from genera of family Asteraceae,such as Petasites [5],Remanthodium [6],Ligularia [7, 8, 9, 10, 11],Senecioneae [12, 13, 14, 15],Robinsonecio [16],Farfarae [17, 18], Achillea [19],Pulicaria [20],Acrisione [21],Arnoglossum [22],and Kleinia [23]. This is the first report on the isolation of oplopane sesquiterpenes from the genus Artemisia,which is of great significance for the chemical diversity of A. gmelinii and genus Artemisia and may also provide new chances for further chemical, bioactive,and taxonomic approaches in the future. Acknowledgment
The authors are very grateful to Prof. Skarma tsogs gnyis of Tibetan Medical College of Qinghai University for the species identification of the plant material.
| [1] | Chinese Academy of Sciences, China Flora Editorial Board, Flora of China, vol. 76 (2), Science Press, 1991, p. 2. |
| [2] | Á. Könczöl, Z. Béni, M.M. Sipos, et al., Antioxidant activity-guided phytochemical investigation of Artemisia gmelinii Webb.ex Stechm.: isolation and spectroscopic challenges of 3,5-O-dicaffeoyl(epi?) quinic acid and its ethyl ester, J. Pharmaceut. Biomed. 56 (2012) 83-89. |
| [3] | M.R. Jia, X.W. Li, Chinese Ethnic Medicine, China Medical Science Press, Beijing, 2005, p. 67. |
| [4] | C.M. Wu, Y.Y. Tu, Study on chemical component of Artemisia gmelinii, Chin. Bull. Bot. 3 (1985) 34-37. |
| [5] | K. Hayashi, Oplopane sesquiterpenes from Petasites palmatus, Phytochemistry 28 (1989) 3373-3376. |
| [6] | T. Motoo, S. Yoshinori, K. Takiguchi, X. Gong, C. Kuroda, Three new bisabolanetype sesquiterpenoids from Cremanthodium rhodocephalum (Asteraceae), Heterocycles 86 (2012) 497-503. |
| [7] | Q. Wang, T.H. Chen, K.F. Bastow, et al., Songaricalarins A-E, cytotoxic oplopane sesquiterpenes from Ligularia songarica, J. Nat. Prod. 76 (2013) 305-310. |
| [8] | H. Nagano, R. Hanai, H. Yamada, et al., Chemical and genetic study of ligularia duciformis and related species in Sichuan and Yunnan Provinces of China, Chem. Biodivers. 9 (2012) 789-805. |
| [9] | X. Gao, W.D. Xie, Z.J. Jia, Four new terpenoids from the roots of Ligularia narynensis, J. Asian Nat. Prod. Res. 10 (2008) 185-192. |
| [10] | M. Tori, M. Fujiwara, Y. Okamoto, et al., New oplopane-type sesquiterpenoids from Ligularia duciformis, Nat. Prod. Commun. 2 (2007) 357-360. |
| [11] | X. Gao, C.J. Lin, W.D. Xie, et al., New oplopane-type sesquiterpenes from Ligularia narynensis, Helv. Chim. Acta 89 (2006) 1387-1394. |
| [12] | R.J.I. Maldonado, A. Arciniegas, A.L. Pérez Castorena, J.L. Villaseñor, R.A. de Vivar, Furanoeremophilanes and other constituents of Pittocaulon bombycophole, Heterocycles 75 (2008) 3035-3042. |
| [13] | P. Joseph-Nathan, J.R. Villagomez, L.U. Romána, J.D. Hernándeza, Oplopanes from the leaves of Senecio mexicanus, Phytochemistry 29 (1990) 977-979. |
| [14] | P. Joseph-Nathan, J.R. Villagomez, M. Rojas-Gardida, L.U. Roman, J.D. Hernandez, Minor oplopanes from Senecio mexicanus, Phytochemistry 28 (1989) 2397-2401. |
| [15] | P. Joseph-Nathan, J.R. Villagomez, L.U. Romána, J.D. Hernándeza, An oplopane from Senecio mexicanus, Phytochemistry 28 (1989) 1207-1209. |
| [16] | A. Arciniegas, A.L. Perez-Castorena, S. Reyes, J. Contreras, R.A. de Vivar, New oplopane and eremophilane derivatives from Robinsonecio gerberifolius, J. Nat. Prod. 66 (2003) 225-229. |
| [17] | Y. Yaoita, N. Suzuki, M. Kikuchi, Studies on the constituents of the flower buds of Tussilago farfara. Part VI. Structures of new sesquiterpenoids from Farfarae flos, Chem. Pharm. Bull. 49 (2001) 645-648. |
| [18] | Y. Yaoita, H. Kamazawa, M. Kikuchi, Studies on the constituents of the flower buds of Tussilago farfara. Part V. Structures of new oplopane-type sesquiterpenoids from the flower buds of Tussilago farfara L., Chem. Pharm. Bull. 47 (1999) 705-707. |
| [19] | K. Anake, L.M. Vieira, J.A. Pereira, M.S. Silva Artur, W. Herz, Further constituents of Achillea ageratum, Phytochemistry 51 (1999) 555-558. |
| [20] | A. San Feliciano, M. Medarde, M. Gordaliza, E. Del Olmo, J.M. Miguel del Corral, Sesquiterpenoids and phenolics of Pulicaria paludosa, Phytochemistry 28 (1989) 2717-2721. |
| [21] | M.A. Aal, F. Bohlmann, T. Sarg, M. El-Domiaty, B. Nordenstam, Oplopane derivatives from Acrisione denticulate, Phytochemistry 27 (1988) 2599-2602. |
| [22] | F. Bohlmann, C. Zdero, R.M. King, H.E. Robinson, Oplopane derivatives from Arnoglossum atriplicifolium, Revista Latinoamericana de Quimica 15 (1984) 11-13. |
| [23] | F. Bohlmann, M. Ahmed, J. Jakupovic, C. Jeffrey, Naturally occurring terpene derivatives. Part 311. Sesquiterpenes from Kleinia species, Phytochemistry 20 (1981) 251-256. |