b Department of Chemistry, Deogiri College, Aurangabad 431005, India
During the past decade,multi-component reactions (MCRs) have become an area of prime interest in synthetic organic chemistry due to their ability in converting more than two components in a single step to complex molecules. MCRs have emerged as efficient,atom economic,time saving and powerful tools in modern synthetic organic chemistry for the synthesis of pharmacologically and biologically important targets as they increase the efficiency of the reaction and avoid the multiple steps along with saving solvents and chemicals. Such reactions allow the formation of new bonds resulting in diverse molecular complexity in a single step [1]. MCRs play a prominent role in modern drug discovery processes [2]. Thus the study of MCRs has become one of the most attractive synthetic strategies preferred by organic chemists in industry and academia.
Derivatives of 1,4-dihydropyridine and polyhydroquinoline heterocyclic scaffolds are important classes of well known Ca2+ channel blockers and constitute the skeletons of drugs used in the treatment of hypertension and cardiovascular diseases [3]. These compounds possess a variety of biological activities including antidiabetic,antitumor,vasodilator,bronchodilator,geroprotective and anti-atherosclerotic properties [4]. They are also explored as antiischemics and in the treatment of Alzheimer’s disease [5]. These facts reflect the remarkable pharmacological and medicinal potential of 1,4-dihydropyridines and polyhydroquinolines as drug candidates of therapeutic significance and as intermediates in organic synthesis. Thus the synthesis of these heterocyclics has become an area of great interest.
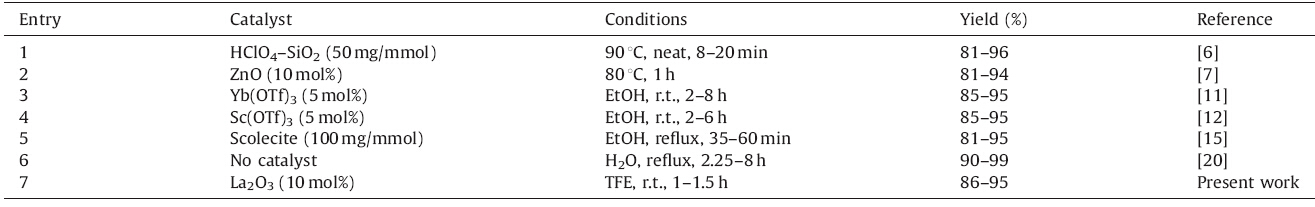
Traditionally 1,4-dihydropyridine and polyhydroquinolines are synthesized by refluxing aldehydes with 1,3-dicarbonyl compound and ammonium acetate catalyzed by acidic or basic catalysts. Literature survey reveals the availability of numerous methods for the synthesis of polyhydroquinoline derivatives using catalysts such as HClO4-SiO2 [6],ZnO [7],CuO [8],nano-Ni [9],t-BuOK [10], Yb(OTf)3 [11],Sc(OTf)3 [12],GuHCl [13],TiO2 [14],scolecite [15], morpholine [16],Zn-VCO3 hydrotalcite [17],magnetic Fe3O4 nanoparticles [18],Mn(III) complex [19],etc. The synthesis in aqueous medium [20] without catalyst although successful, requires longer reaction time (2.25-8 h). Thus many of the existing strategies suffer from harsh reaction conditions,use of stoichiometric and/or relatively expensive reagents,long reaction time, unsatisfactory yield of products,etc. Some of these methods,for example microwave or ultrasound assisted synthesis [21],require additional equipment such as a microwave oven or a sonication bath. Ionic liquids [22] have also been used for clean chemical reactions replacing volatile organic solvents. But they suffer from inherent problems in separation and tedious workup is often involved. Consequently,research continues in the development of novel and facile protocols for the synthesis of polyhydroquinolines with improved operational simplicity,economic viability,reusability and milder reaction conditions.
Recently,the characteristic features of fluorinated solvents such as high polarity,strong hydrogen accepting ability and low boiling points prompted their applications in organic synthesis. 2,2,2- Trifluoroethanol (TFE) is a low boiling (bp 74 8C),colorless and water miscible organic solvent. It has become one of the more widely used fluorinated solvents. It exhibits stronger acidic character and higher stability than the non-fluorinated alcohol due to the presence of the electronegative trifluoromethyl group. TFE is commonly used as a fluorinated alcohol on commercial scale manufacture processes. TFE has been used as an alternative to metal-assisted syntheses including several multi-component reactions such as the synthesis of dibenzoc, eazepinones,1,3,4- trisubsituted pyrazoles,reductive alkylation,etc. [23].

|
Download:
|
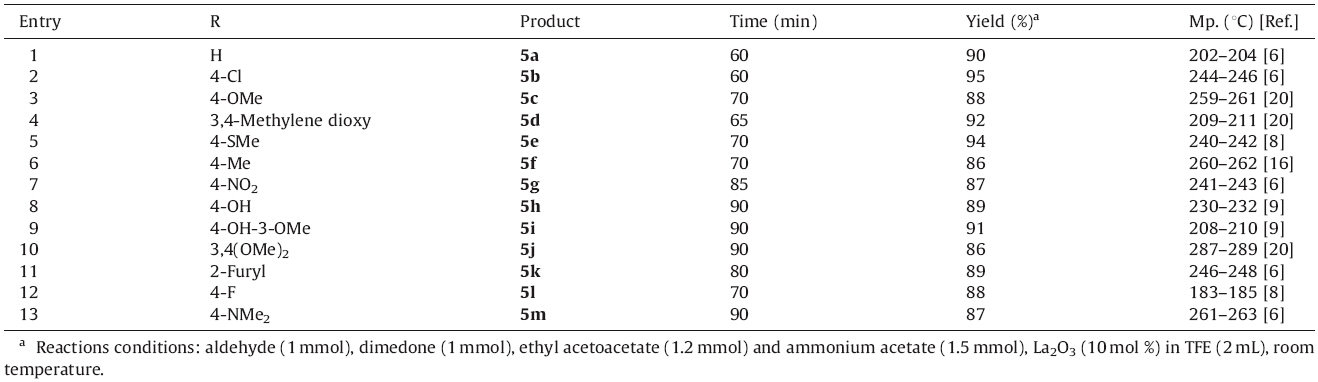
| Scheme 1.Synthesis of Hantzsch polyhydroquinoline derivatives by using La2O3/TFE. | |
Several inorganic metal oxides,such as ZnO,CuO,Al2O3,TiO2, etc. act as efficient heterogeneous catalysts due to their Lewis acid- base characters and ability to provide large surface area for adsorption of organic molecules [24]. Lanthanum oxide (La2O3), also known as Lanthana,is a white,odorless,and large band gap (5.5 eV) rare earth metal oxide with a high dielectric constant (e = 27). It is thermally stable (melting point 2315 8C),cheap, readily available and insoluble in water. Consequently it can be employed in organic synthesis as an effective heterogeneous catalyst. In recent years,lanthanum oxide and its composites came into limelight as catalysts in organic synthesis such as carbonylation of glycerol [25],C-N coupling reactions [26],Heck reaction [27],Wittig reaction [28],etc. In continuation of our efforts in the development of new synthetic routes for the synthesis of heterocyclic compounds using reusable heterogeneous catalysts [29],we report the combination of TFE/La2O3 as an efficient tool for the one-pot,four-component synthesis of polyhydroquinolines at room temperature in excellent yields (Scheme 1). 2. Experimental
All the chemicals were purchased from SD fine chemicals Ltd. and used without further purification. The synthesized polyhydroquinoline derivatives were confirmed on the basis of spectral data and comparison of their physical constants to those reported in literature. Melting points were measured in capillaries open at one end and were uncorrected. The progress of reaction was monitored by thin-layer chromatography (TLC) analysis in 30% EA: Hexane. 1H NMR spectra were recorded in CDCl3 on a 400 MHz Varian spectrophotometer using tetramethylsilane (TMS) as an internal standard. Infrared (IR) spectra were recorded on a Shimadzu FTIR spectrometer using KBr pellets. Samples were analyzed for exact mass on a Shimadzu mass analyzer.
General procedure for the synthesis of Hantzsch polyhydroquinoline derivatives: In a typical condensation reaction,a mixture of aldehyde (1 mmol),dimedone (1 mmol),ethyl acetoacetate (1.2 mmol),ammonium acetate (1.5 mmol) and La2O3 (10 mol%) in TFE (2 mL) was magnetically stirred at room temperature for appropriate time as specified in Table 1. After the completion of the reaction as monitored by TLC (30% EA: Hex) analysis,the reaction mixture was diluted with hot TFE,and the catalyst was filtered off. The filtrate was concentrated and the crude was purified by recrystallization from ethanol to afford the pure polyhydroquinoline derivatives.
The structures of the synthesized products were confirmed by comparison of their melting points with authentic values reported in literature and spectral techniques-1H NMR,IR,elemental analysis and ESMS. The spectral data of representative compounds are described below: Ethyl-1,4,5,6,7,8-hexahydro-2,7,7-trimethyl-5-oxo-4-phenylquinoline- 3-carboxylate (5a): Faint yellow solid,Mp 202-204°C, 1H NMR (400 MHz,CDCl3:δ0.95 (s,3H,CH3),1.1 (s,3H,CH3),1.2 (t,3H,CH3,2.15-2.35 (m,4H),2.4 (s,3H),4.05 (q,2H),5.03 (s,1H),6.02 (brs,1H,NH),7.1-7.4 (m,5H). IR (KBr,cm-1): n 3285.88,3082.38,2954.11,1697.43,1609.67. Anal. Calcd. for C21H25NO3: C,74.31,H,7.42,N,4.13. Found: C,72.43,H,7.029, N,3.97.
| Table 1 TFE mediated La2O3 catalyzed synthesis of polyhydroquinoline derivatives. |
Ethyl-4-(4-chlorophenyl)-1,4,5,6,7,8-hexahydro-2,7,7-trimethyl- 5-oxoquinoline-3-carboxylate (5b): Yellow solid,Mp 244- 246 8C,1H NMR (400 MHz,CDCl3:δ0.92 (s,3H),1.07 (s,3H),1.2 (t, 3H),2.15-2.30 (m,4H),2.4 (s,3H),4.04 (q,2H),5.03 (s,1H),5.82 (brs,1H,NH),7.15-7.20 (d,2H,J = 8.4 Hz),7.20-7.30 (d,2H, J = 8.4 Hz). IR (KBr,cm-1): n 3274.31,3077.56,2958.93,1705.15, 1647.28,1602.91. Anal. Calcd. for C21H25NO3: C,67.46,H,6.47,N, 3.75. Found: C,67.55,H,6.38,N,3.68,ES-MS: 396.03 [M+Na]+.
Ethyl-4-(benzod1, 3dioxol-6-yl)-1,4,5,6,7,8-hexahydro- 2,7,7-trimethyl-5-oxoquinoline-3-carboxylate (5d): Faint yellow solid,Mp 209-211 8C,1H NMR (400 MHz,CDCl3:δ0.93 (s,3H, CH3,1.07 (s,3H,CH3,1.23 (t,3H),2.13-2.35 (m,4H),2.35 (s,3H), 4.07 (q,2H),4.98 (s,1H),5.87 (s,2H),6.08 (brs,1H,NH),6.6-6.83 (m,3H). IR (KBr,cm-1): n 3273.34,3077.56,3198.11,1696.47, 1604.84,1031.00,884. Anal. Calcd. for C21H25NO5: C,68.91,H, 6.57,N,3.65. Found: C,66.54,H,6.415,N,3.34,ES-MS: 406.04 [M+Na]+.
Ethyl-1,4,5,6,7,8-hexahydro-2,7,7-trimethyl-4-(4-(methylthio) phenyl)-5-oxoquinoline-3-carboxylate (5e): Yellow solid,Mp 240-242 8C,1H NMR (400 MHz,CDCl3:δ0.92 (s,3H,CH3,1.1 (s,3H,CH3,1.21 (t,3H),2.1-2.3 (m,4H),2.38 (s,3H),2.42 (s,3H), 4.07 (q,2H),5.0 (s,1H),6.02 (brs,1H,NH),7.05-7.20 (d,2H, J = 7.2 Hz),7.20-7.35 (d,2H,J = 7.2 Hz). IR (KBr,cm-1): n 3277.20, 3077.56,2958.93,1700.32,1601.95,1487.18,1367.59,1279.82, 1231. Anal. Calcd. for C21H25NO5S: C,68.54,H,7.06,N,3.63. Found: C,68.47,H,6.917,N,3.53,ES-MS: 408.02 [M+Na]+. 3. Results and discussion
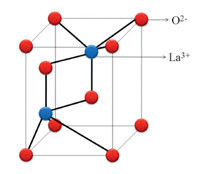
The acidic nature of fluorinated organic solvents primarily arises from the fluorine atoms. The presence of an electronegative trifluoromethyl group in TFE affords it strong acidic character. Lanthanum oxide has temperature dependent crystal structure [30]. At ambient temperature,it has a hexagonal crystal structure in which the La3+ ion (Lewis acid) is surrounded by 7 co-ordinate group of O2- ions (Fig. 1).
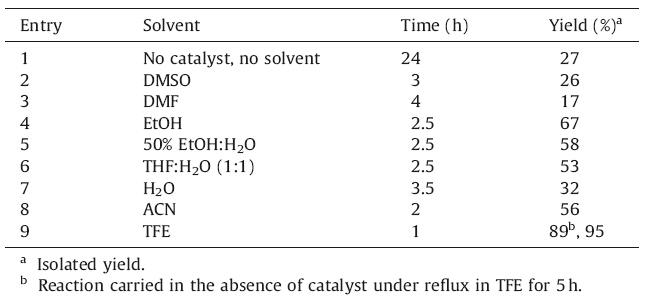
At higher temperature,it becomes a cubic crystal. These basic structural features prompted us to use the combination of these two in organic synthesis for the construction of heterocyclic compounds such as Hantzsch polyhydroquinoline derivatives. To optimize the reaction conditions and select proper solvent,the reaction of 4-Cl-benzaldehyde (1 mmol),dimedone (1 mmol), ethyl acetoacetate (1.2 mmol) and ammonium acetate (1.5 mmol) using La2O3 (10 mol%) catalyst in 2 mL of respective solvent was chosen as the model condensation reaction whose results are summarized in Table 2.
Initially the reaction was tried at room temperature in the absence of any catalyst and solvent. When the reaction was carried at room temperature in the absence of catalyst,it could not complete even after a long time (24 h) (Table 2,entry 1). Then we continued to optimize the model condensation using various solvents and their combinations. The use of solvents such as DMSO or DMF at ambient temperature resulted in low yield of the corresponding polyhydroquinolines after 3-4 h. Binary solvent systems such as EtOH:H2O (1:1) or THF:H2O (1:1) could not improve the results. Acetonitrile could accomplish moderate yield (56%). From the literature survey,ethanol is a common solvent employed for the synthesis of 1,4-dihydropyridine and polyhydroquinolines. When the reaction was carried in ethanol,the yield was improved and the reaction time was shortened to some extent. However,excellent yields with short reaction time were possible with the use of 2,2,2-trifluoroethanol (TFE). To study the temperature effect in the TFE-mediated synthesis of polyhydroquinolines, a reaction was carried in the absence of catalyst by simply refluxing a mixture of 4-chlorobenzaldehyde,dimedone, ethyl acetoacetate and ammonium acetate in which a good yield of the corresponding polyhydroquinoline (89%) was obtained but the reaction required 5 h for completion. Furthermore,to determine the amount of the catalyst in this reaction,the reactions were carried with different concentrations of La2O3. The rise in catalyst concentration from 10 to 15 or 20 mol% could neither enhance the yield of product nor reduce the time to below 1 h. Thus,excellent results were obtained with 10 mol% of La2O3 in TFE at room temperature in terms of yield as well as time. The scope and generality of the one-pot,four-component synthesis of polyhydroquinoline derivatives through the Hantzsch reaction was verified with different aldehydes under these optimized conditions. Almost all aldehydes,possessing both electron-donating as well as electron withdrawing groups along with heterocyclic aldehydes reacted smoothly with clean reaction profile to afford the corresponding polyhydroquinoline derivatives.
|
Download:
|
| Fig. 1.Structure of La2O3. | |
| Table 2 Solvent screening for La2O3 catalyzed synthesis of polyhydroquinolines. |
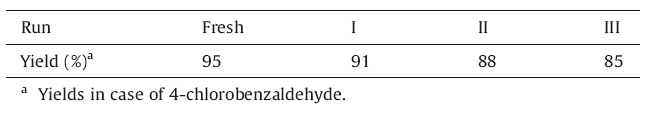
No significant substituent effect was observed in terms of reaction time and yield of the product. Furthermore,reusability study of the catalyst showed good results. 4-Chlorobenzaldehyde gave 95%,91%,88%,85% yield of the ethyl 4-(4-chlorophenyl)- 1,4,5,6,7,8-hexahydro-2,7,7-trimethyl-5-oxoquinoline-3-carboxylate after fresh,1st,2nd and 3rd run,respectively. The catalyst can be recycled and reused several times without much loss of catalytic activity (Table 3).
Thus,the present protocol tolerates different functional groups, such as methoxy,hydroxyl,halide,etc.,and smoothly affords the polyhydroquinolines in short reaction time at room temperature in excellent yields. A comparative study of the effect of La2O3/TFE combination with some of the literature methods for the synthesis of polyhydroquinolines is summarized in Table 4.
| Table 3 Reusability of La2O3 catalyst. |
| Table 4 Comparison of La2O3/TFE combination with literature methods for the synthesis of polyhydroquinolines. |
Thus inthe presentwork,wehavedemonstrated the utility of the combination of lanthanum oxide and trifluoroethanol (La2O3/TFE) for the synthesis of Hantzsch polyhydroquinolines from aromatic aldehydes,dimedone,ethylacetoacetate and ammonium acetate at room temperature. The presence of Lewis acidic sites (La3+) in the catalyst and trifluoromethyl group in TFE increased Lewis acidity of the combination of La2O3/TFE system,which was sufficient enough to catalyze the reaction at ambient temperature affording high yield of products in short reaction time. Use of heterogeneous catalyst, tolerance to various substituents,easy separation,short reaction time and high yield are the significant advantages associated with the present protocol,which make it an attractive strategy for the synthesis of polyhydroquinolines. Thus,the present protocol highlights and explores not only the applications of 2,2,2- trifluoroethanol as a powerful solvent in organic synthesis but also the emerging utility of La2O3 as a heterogeneous catalyst for the synthesis of heterocyclic compounds. We strongly believe that the combination will find extensive applications in future for the synthesis of heterocyclic compounds. Acknowledgments
Authors are thankful to the Principal,Shri Muktanand College, Gangapur (MS) India and Principal,Deogiri College Aurangabad (MS) India for encouraging us to carry out this work. One of the authors (SUT) thanks University Grants Commission for Financial assistance under a Minor Research Project [No. 47-283/12(WRO)].
| [1] | V A. Orru, E. Ruijter, Synthesis of Heterocycles via Multicomponent Reactions, I, Springer, Berlin, Heidelberg.2010. |
| [2] | C. Hulme, V. Gore, Multi-component reactions: emerging chemistry in drug discovery from xylocain to crixivan, Curr. Med. Chem. 10 (2003) 51-80. |
| [3] | Y. Nishiya, N. Kosaka, M. Uchii, S. Sugimoto, A potent 1,4-dihydropyridine L-type calcium channel blocker, benidipine, promotes osteoblast differentiation, Calcif. Tissue Int. 70 (2002) 30-39. |
| [4] | (a) K. Sirisha, G. Achaiah, V.M. Reddy, Facile synthesis and antibacterial, antitubercular, and anticancer activities of novel 1,4-dihydropyridines, Arch. Pharm. 343 (2010) 342-352; (b) R. van der Lee, M. Pfaffendorf, P.A. van Zwieten, The differential time courses of the vasodilator effects of various 1,4-dihydropyridines in isolated human small arteries are correlated to their lipophilicity, J. Hypertens. 18 (2000) 1677-1682. |
| [5] | S. Yasar,M. Corrada, R. Brookmeyer, C. Kawas, Calcium channel blockers and risk of AD: the Baltimore longitudinal study of aging, Neurobiol. Aging 26 (2005) 157-163. |
| [6] | M. Maheswara, V. Siddaiah, G. Lakishmi, V. Damu, C.V. Rao, An efficient one-pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation using a heterogeneous catalyst under solvent-free conditions, Arkivoc ii (2006) 201-206. |
| [7] | F.M. Moghaddam, H. Saeidian, Z. Mirjafary, A. Sadeghi, Rapid and efficient one-pot synthesis of 1,4-dihydropyridine and polyhydroquinoline derivatives through the Hantzsch four component condensation by zinc oxide, J. Iran. Chem. Soc. 6 (2009) 317-324. |
| [8] | J. Safaei-Ghomi, M.A. Ghasemzadeh, Nanocrystalline copper(Ⅱ) oxide-catalyzed one-pot four component synthesis of polyhydroquinoline derivatives under solvent-free conditions, J. Nanostruct. 1 (2012) 243-248. |
| [9] | S.B. Sapkal, K.F. Shelke, B.B. Shingate, M.S. Shingare, Nickel nanoparticle-catalyzed facile and efficient one-pot synthesis of polyhydroquinoline derivatives via Hantzsch condensation under solvent-free conditions, Tetrahedron Lett. 50 (2009) 1754-1756. |
| [10] | A. Debache, L. Chouguiat, R. Boulcina, B. Carbonib, A one-pot multi-component synthesis of dihydropyrimidinone/thione and dihydropyridine derivatives via Biginelli and Hantzsch condensations using t-BuOK as a catalyst under solvent-free conditions, Open Org. Chem. J. 6 (2012) 12-20. |
| [11] | L.M. Wang, J. Sheng, L. Zhang, et al., Facile Yb(OTf)3 promoted one-pot synthesis of polyhydroquinoline derivatives through Hantzsch reaction, Tetrahedron 61 (2005) 1539-1543. |
| [12] | J.L. Donelson, R.A. Gibbs, S.K. De, An efficient one-pot synthesis of polyhydroquinoline derivatives through the Hantzsch four component condensation, J. Mol. Catal. A: Chem. 256 (2006) 309-311. |
| [13] | S.M. Baghbanian, S. Khaksar, S.M. Vahdat, M. Farhang, M. Tajbakhsh, One-step, synthesis of Hantzsch esters and polyhydroquinoline derivatives using new organocatalyst, Chin. Chem. Lett. 21 (2010) 563-567. |
| [14] | S. Abdolmohammadi, Simple route to indeno[1,2-b]quinoline derivatives via a coupling reaction catalyzed by TiO2 nanoparticles, Chin. Chem. Lett. 24 (2013) 318-320. |
| [15] | L.S. Gadekar, S.S. Katkar, S.R. Mane, B.R. Arbad, M.K. Lande, Scolecite catalyzed facile and efficient synthesis of polyhydroquinoline derivatives through Hantzsch multi-component condensation, Bull. Korean Chem. Soc. 30 (2009) 2532-2534. |
| [16] | M.M. Heravi, M. Zakeri, S. Pooremamy, H.A. Oskooie, Clean and efficient synthesis of polyhydroquinoline derivatives under solvent-free conditions catalyzed by morpholine, Synth. Commun. 1 (2011) 113-120. |
| [17] | R. Pagadala, S. Maddila, V.D.B.C. Dasireddy, S.B. Jonnalagadda, Zn-VCO3 hydrotalcite: a highly efficient and reusable heterogeneous catalyst for the Hantzsch dihydropyridine reaction, Catal. Commun. 45 (2014) 148-152. |
| [18] | M. Nasr-Esfahani, S.J. Hoseini, M. Montazerozohori, R. Mehrabi, H. Nasrabad, Magnetic Fe3O4 nanoparticles: efficient and recoverable nanocatalyst for the synthesis of polyhydroquinolines and Hantzsch 1,4-dihydropyridines under solvent-free conditions, J. Mol. Catal. A: Chem. 382 (2014) 99-105. |
| [19] | E. Mosaddegh, A. Hassankhani, An efficient and rapid Mn(Ⅲ) complex catalyzed synthesis of polyhydropyridine derivatives via Hantzsch four component condensation, Arab. J. Chem. 5 (2012) 315-318. |
| [20] | B.P. Bandgar, P.E. More, V.T. Kamble, J.V. Totre, Synthesis of polyhydroquinoline derivatives under aqueous media, Arkivoc xv (2008) 1-8. |
| [21] | (a) N.K. Ladani, D.C. Mungra, M.P. Patel, R.G. Patel, Microwave assisted synthesis of novel Hantzsch 1,4-dihydropyridines, acridine-1,8-diones and polyhydroquinolines bearing the tetrazolo[1,5-a]quinoline moiety and their antimicrobial activity assess, Chin. Chem. Lett. 22 (2011) 1407-1410; (b) A. Kumar, R.A. Maurya, Efficient synthesis of Hantzsch esters and polyhydroquinoline derivatives in aqueous micelles, Synlett 6 (2008) 883-885. |
| [22] | M. Tajbakhsh, H. Alinezhad, M. Norouzi, S. Baghery, M. Akbari, Protic pyridinium ionic liquid as a green and highly efficient catalyst for the synthesis of polyhydroquinoline derivatives via Hantzsch condensation in water, J. Mol. Liq. 177 (2013) 44-48. |
| [23] | (a) V.P. Mehta, S.G. Modha, E. Ruijter, et al., A microwave-assisted diastereoselective multicomponent reaction to access dibenzo[c,e]azepinones: synthesis and biological evaluation, J. Org. Chem. 76 (2011) 2828-2839; (b) H. Alinezhad, M. Tajbakhsh, M. Zare, Catalyst-free one-pot synthesis of 1,4,5-trisubstituted pyrazoles in 2,2,2-trifluoroethanol, J. Fluor. Chem. 132 (2011) 995-1000. |
| [24] | J.A. Kurzman, L.M. Misch, R. Seshadri, Chemistry of precious metal oxides relevant to heterogeneous catalysis, Dalton Trans. 42 (2013) 14653-14667. |
| [25] | L. Wang, Y. Ma, Y. Wang, S. Liu, Y. Deng, Efficient synthesis of glycerol carbonate from glycerol and urea with lanthanum oxide as a solid base catalyst, Catal. Commun. 12 (2011) 1458-1462. |
| [26] | S.N. Murthy, B. Madhav, V.P. Reddy, Y.V.D. Nageswar, A new, efficient and recyclable lanthanum(Ⅲ) oxide-catalyzed C-N cross-coupling, Adv. Synth. Catal. 352 (2010) 3241-3245. |
| [27] | A. Cwik, Z. Hell, F. Figueras, Palladium/magnesium-lanthanum mixed oxide catalyst in the Heck reaction, Adv. Synth. Catal. 348 (2006) 523-530. |
| [28] | M.L. Kantam, K.B. Shiva Kumar, V. Balasubramanyam, G.T. Venkanna, F. Figueras, One-pot Wittig reaction for the synthesis of α,β-unsaturated esters using highly basicmagnesium/lanthanummixed oxide, J.Mol. Catal.A: Chem.321(2010) 10-14. |
| [29] | (a) S.U. Tekale, A.B. Tekale, N.S. Kanhe, S.V. Bhoraskar, R.P. Pawar, Nano-particulate aluminiumnitride/Al: an efficient and versatile heterogeneous catalyst for the synthesis of Biginelli scaffolds, Am. Inst. Phys. Conf. Proc. 1393 (2011) 275-276; (b) S.U. Tekale, S.S.Shisodia, S.S.Kauthale, et al.,MicronparticlesofAlN/Al: efficient, novel, and reusable heterogeneous catalyst for the synthesis of bis(indolyl)-methanes, Synth. Commun. 43 (2013) 1849-1858. |
| [30] | R.W.G. Wyckoff, Crystal Structures: Inorganic Compounds RXn, RnMX2, RnMX3, Interscience Publishers, New York, 1963. |