Functionalized anilines are important intermediates for pharmaceuticals,polymers,herbicides,and other substances and fine chemicals. Among them,fluoroanilines (FA) are important building blocks for the synthesis of numerous bioactive products [1, 2, 3, 4]. For their importance,the selective hydrogenation of aromatic nitro compounds to the corresponding anilines is an industrially important reaction [5, 6, 7, 8, 9].
A broad range of catalysts for the hydrogenation of halogenated nitrobenzenes has been well-studied,such as Pt [5],Pd [6],Rh [7] and Ni [10] catalysts. Depending on the halogens and their position relative to the nitro group in the halogenated nitrobenzene, hydrogenolysis of the C-X bond and nucleophilic substitution of the C-N coupling reaction leading to the formation of nitro- and/or amino-diphenylamine,may happen varying from negligible to 100% [11, 12]. Great efforts have been made to improve the hydrogenation selectivity,such as choosing efficient supports [13, 14, 15],adding promoters [16, 17],poisoning [18] and alloying [19].
However,on one hand,catalysts such as Pd/C with high intrinsic activity suffer from low selectivity. On the other hand,catalysts of higher selectivity obtained by poisoning or alloying require high reaction temperature and high H2 pressure because of their lower intrinsic activity. Recently,more and more attention has been focused on the noble metal/non-noble metal oxide NPs catalysts as potential catalysts for the hydrogenation of halogenated nitrobenzenes. These catalysts often display a higher performance than their monometallic counterparts. In Coq’s study [20],the catalytic hydrogenation properties of Pt/TiO2and Pt/Al2O3catalysts usingpchloronitrobenzene (p-CNB) as a model substrate were compared and it was found that the ratio of the hydrogenation activity to hydrodechlorination activity over the Pt/TiO2catalyst was 10-fold higher than that over the Pt/Al2O3catalyst. More recently,Wang [21] reported that the assembled Ru/SnO2 catalysts exhibited excellent catalytic activity in the hydrogenation ofo-chloronitrobenzene (o-CNB) to the correspondingo-chloroaniline (o-CAN), and the catalytic activity surpassed that of all previously reported Ru catalysts. And the selectivity of theo-CAN was in agreement with the best results reported on a colloidal Ru catalyst so far. Notably,the non-noble metal oxide appears necessary to provide a desired chemical interface between the NPs and the reaction media. Herein,the catalytic activity of Pd/SnO2/C catalysts was investigated for the liquid-phase hydrogenation of 2,4-difluoronitrobenzene (DFNB) to 2,4-difluoroaniline (DFAN) under mild reaction conditions,and some new insights about the catalytic properties of Pd/SnO2/C were revealed. 2. Experimental
The Pd/SnO2/C catalysts were prepared in two steps: first deposition of the SnO2onto the carbon followed by the deposition of Pd. Bu3SnCl was then charged into an aqueous slurry of the carbon support to obtain a SnO2loading of 10 wt%,then the pH value was adjusted to 8-9 under stirring. Subsequently,the solid was collected by filtration,washed,dried and then calcined in N2 gas stream at 773 K for 4 h. Then an aqueous solution of H2PdCl4 (0.05 gmetal/mL) was added into an aqueous slurry of the SnO2/C supports to obtain a Pd loading of 2 wt%. After stirring,the solution pH value of 8-9 was reached,then the precipitated Pd(OH)2was reduced by hydrazine hydrate. Finally,the slurry was washed and dried under vacuum at 383 K for 10 h,and kept for use. The Pd/C heterogeneous catalyst with 2 wt% Pd loading was also prepared using the same procedures. A classical Pd/SnO2 heterogeneous catalyst with 2 wt% Pd loading was also prepared by the traditional wetness impregnation method using H2PdCl4 and prepared SnO2 supports as precursors. The SnO2 support was prepared by neutralizing the prepared SnO2sol with an aqueous solution of NaOH and drying the SnO2precipitate at 383 K. The catalyst was calcined at 573 K for 2 h and reduced with hydrogen at 523 K for 3h.
TEM study was carried out in a Philips-FEI Tecnai G2 F30 S-Twin instrument. XRD measurements of the catalyst samples were performed on a PANalytical-X’Pert PRO generator. XPS was acquired with a Kratos AXIS Ultra DLD spectrometer. H2 TPD experiments were performed by first reducing the samplein situat 200°C. Then the sample was swept with pure Ar to remove physiosorbed and/or weakly bound species. The TPD spectra were recorded by TCD.
Liquid phase hydrogenation of DFNB was conducted as follows: 150 mL of ethanol,10.0 g of DFNB,and 0.1 g of the catalyst were mixed in a 500-mL steel autoclave. Air in the autoclave was purged by hydrogen,and then the reaction proceeded at the required temperature (363 K) and at 1 MPa of 99.99% pure hydrogen. The selectivity of the hydrogenation of DFNB was calculated using the following equations:
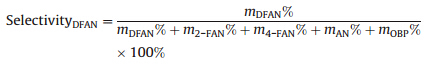
where 2-FAN represents 2-fluoroaniline; 4-FAN represents 4-fluoroaniline; AN represents aniline; OBP represents the amount of azoxybenzene (AOB),azobenzene (AB),hydrazobenzene (HAB),Nphenylhydroxylamine (PHA),nitrosobenzene (NSB),aminodiphenylamine (ADPA) and nitrodiphenylamine (NDPA) in the mixture. 3. Results and discussion
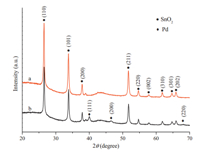
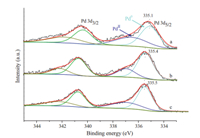
Fig. 1a showed that the Pd NPs were highly dispersed on the SnO2NPs. As indicated in Fig. 1b,there were two kinds of lattice planes that were identified with the NPs,which correspond to the (110) plane of SnO2and the (200) plane of Pd. Generally,the activation energy for heterogeneous nucleation is lower than that for homogeneous nucleation [22],which results in Pd'atoms depositing on SnO2surfaces rather than forming discrete Pd NPs on carbon support. The size of Pd particles was distributed mainly in the range of 3-5 nm with an average size of Pd NPs being about 4 nm (Fig. 1a). In the XRD patterns shown in Fig. 2,the obvious crystal planes of the SnO2were observed for both SnO2/C and Pd/ SnO2/C samples. The peaks corresponding to Pd NPs at 2θ= 40.13° and 46.96° relative to the (111) and (220) planes,respectively, could be observed (Fig. 2b). As shown in Fig. 3,the areas of the H2 TPD peaks for the Pd/SnO2/C catalyst were much larger than those for the Pd/C and Pd/SnO2catalysts,which may be attributed to the hydrogen spillover effect in Pd/SnO2/C. The enhancement in the amount of chemosorbed hydrogen may be beneficial for improving catalytic activity [23]. The XPS core level spectra of Pd 3d of the various catalysts are shown in Fig. 4. The Pd 3d peaks were detected at a range of binding energy (BE) of 335.1-335.5 eV for the Pd/C,Pd/SnO2 and Pd/SnO2/C catalysts,which is typical for metallic Pd0[24]. In addition,the binding energy of Pd 3d5/2 detected at 336.5-337.1 eV was attributed to the presence of palladium in the form ofPdIIO. The presence ofPdIIO on the surface was ascribed to the easy oxidation of Pd upon contact with air at room temperature. It was observed that in the Pd/SnO2and Pd/ SnO2/C samples,the position of the Pd0 3d5/2 signal presented a slightly shift of 0.3 eV and 0.4 eV,respectively,compared with that of the Pd/C sample,which presented at a binding energy of 335.1 eV. Upon the addition of the SnO2,the electronic structure of the surface Pd atoms was modified.

|
Download:
|
| Fig. 1. (a) TEM image of Pd/SnO2/C and the crystallite size distribution and (b) HRTEM image of Pd/SnO2/C. | |
|
Download:
|
| Fig. 2. XRD patterns of (a) SnO2/C and (b) Pd/SnO2/C. | |
|
Download:
|
| Fig. 3. H2 TPD profiles of (a) Pd/SnO2/C, (b) Pd/SnO2, and (c) Pd/C | |
|
Download:
|
| Fig. 4. Pd 3d photoemission core-level spectra for (a) Pd/C, (b) Pd/SnO2and (c) Pd/ SnO2/C. | |
When the hydrogenation of nitrobenzene was carried out in organic solvents,several intermediates were frequently produced and accumulated during the reaction [25, 26, 27, 28, 29]. In the case of fluoronitrobenzene,a possible side reaction is the nucleophilic substitution C-N coupling reaction that leads to the formation of amino- and/or nitro-diphenylamine. Interestingly,the production of these intermediates was inhibited when hydrogenation of nitrobenzene was catalyzed using a Pd/SnO2/C catalyst,and the results can be found in Table 1. Throughout the whole reaction process,100% DFAN selectivity was achieved and no undesired intermediates accumulated during the reaction. For the Pd/C,the selectivity of DFAN was only 96.47%. In addition,the carbon supported Pd/SnO2 catalyst exhibited unexpected activity. Although the high selectivity of DFAN (99.96%) was also achieved on the Pd/SnO2catalyst,the catalytic activity decreased remarkably. The Pd/SnO2catalyst presented a lower activity in converting DFNB when compared with the Pd/SnO2/C catalyst,which might be attributed to the fact that the latter possessed more accessible surface areas. Using the SnO2/C catalysts,the hydrogenation reaction could not proceed because of the absence of the sites activating hydrogen.
| Table 1 Catalytic results of the hydrogenation of DFNB.a |
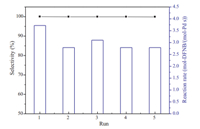
The stability of Pd/SnO2/C was also examined; the catalysts were reused directly without any treatment after precipitated and separated from the reaction solution. It was noted that the catalysts should be immersed in the solution during the recycling process to avoid the oxidation of the active Pd species. The Pd/ SnO2/C presented as relatively stable under the reaction conditions. Although the activity decreased slightly,there is no significant decrease in selectivity for at least 5 cycles (Fig. 5).
|
Download:
|
| Fig. 5. The recycling results for Pd/SnO2/C catalyst in the hydrogenation of DFNB. | |
Coq [20] has reported that modulating the interactions between metal particles and supports could improve the selectivity,and proposed that suboxide TiOxspecies migrating on the Pt particles could polarize the NO bond in p-CNB and would then be responsible for promoting the hydrogenation activity. With respect to the effect of SnO2as a support of Pd,it can be suggested that in our case the effect of SnO2is related to the acidity of the Sn ions (Lewis acidity),which activate the NO group by enhancing its positive charge. Another positive effect could also result from the formation of the electron-donor levels (oxygen vacanciesV') or the increase of coordinatively unsaturated Sn2+ or Sn4+species at the surface of SnO2in a reducing atmosphere such as H2 [30],which would increase the free electron concentration. These unsaturated Sn2+ or Sn4+surface species support the Pd NPs,may activate the polar NO groups of DFNB and coordinate with the NH2 groups of DFAN,thereby promote the hydrogenation of DFNB and suppress both the hydrodehalorination and coupling side reactions. Combining the XPS and H2 TPD analysis,the significantly improved catalytic activity of Pd/SnO2/C catalysts was likely resulted from the enhancement in the amount of active species and the strong interactions between Pd and SnO2 NPs,which lead to the high activity of Pd active species. However,it should be noted that a slight binding energy shift of 0.4 eV on the Pd0 3d5/2 peak could be detected,which strongly indicates an electron transfer from Pd to SnO2due to its strong electron withdrawing ability,which may suppress the hydrogenolysis of C-F bond. 4. Conclusion
The prepared Pd/SnO2/C catalyst displays a high performance (selectivity,activity and stability) for the hydrogenation of DFNB to the corresponding DFAN. The enhance performance may partially be derived from the coordination action between the NH2 groups and coordinatively unsaturated Sn2+ or Sn4+species at the surface of SnO2to form Pd-Snn+ion pairs. These oxygen vacancies of SnO2 produced under reductive atmosphere are believed to account for the high catalytic activity and selectivity in the hydrogenation of DFNB. In addition,the upward shift of Pd'binding energy indicates an electron deficiency of Pd due to an electron transfer from Pd to SnO2,which may suppress the hydrogenolysis of C-F bonds. The excellent catalytic performance and good recyclability of the Pd/ SnO2/C catalyst will make it attractive for fundamental research and practical applications. Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 20976164,21176221 and 21136001), National Basic Research Program of China (973 Program) (Nos. 2011CB710803 and 2013CB733500).
| [1] | R. Filler, Y. Kobayashi, L.M. Yagupolskii, Organofluorine Compounds in Medicinal Chemistry and Biomedical Applications. Elsevier, Amsterdam, 1993. |
| [2] | R.E. Banks, B.E. Smart, J.C. Tatlow, Organofluorine Chemistry: Principles and Commercial Applications, Springer, New York, 1994. |
| [3] | J.T. Welch, S. Eswarakrishnan, Flourine in Bioorganic Chemistry, Wiley, New York, 1991. |
| [4] | A. Becker, Inventory of Industrial Fluoro-Biochemicals, Eyrolles, Paris, 1996. |
| [5] | L. Červený, I. Paseka, V. Stuchlý, et al., Hydrogenation of aromatic nitro compounds on copper-modified platinum catalysts, Collect. Czech. Chem. Commun. 47 (1982) 853-857. |
| [6] | R. Baltzly, A.P. Phillips, The catalytic hydrogenolysis of halogen compounds, J. Am. Chem. Soc. 68 (1946) 261-265. |
| [7] | W. Dunworth, F. Nord, Investigations on the mechanism of catalytic hydrogenations. 1 XVII. Reductions with rhodium on activated carbon, J. Am. Chem. Soc. 74 (1952) 1459-1462. |
| [8] | C.F. Winans, Nickel as a catalyst for the hydrogenation of aromatic halogen compounds, J. Am. Chem. Soc. 61 (1939) 3564-3565. |
| [9] | A. Furst, R.C. Berlo, S. Hooton, Hydrazine as a reducing agent for organic compounds (catalytic hydrazine reductions), Chem. Rev. 65 (1965) 51-68. |
| [10] | W.W. Lin, H.Y. Cheng, J. Ming, et al., Deactivation of Ni/TiO2 catalyst in the hydrogenation of nitrobenzene in water and improvement in its stability by coating a layer of hydrophobic carbon, J. Catal. 291 (2012) 149-154. |
| [11] | R.A. Johnstone, A.H. Wilby, I.D. Entwistle, Heterogeneous catalytic transfer hydrogenation and its relation to other methods for reduction of organic compounds, Chem. Rev. 85 (1985) 129-170. |
| [12] | G. Cordier, J.M. Grosselin, R.-M. Ferrero, High selectivities in hydrogenation of halogenonitro-benzenes on Pd, Pt or raney nickel as catalysts, Ind. Chem. Libr. 8 (1996) 336-342. |
| [13] | M.H. Liu, W.Y. Yu, H.F. Liu, Selective hydrogenation of o-chloronitrobenzene over polymer-stabilized ruthenium colloidal catalysts, J. Mol. Catal. A: Chem. 138 (1999) 295-303. |
| [14] | W.X. Tu, H.F. Liu, Y. Tang, The metal complex effect on the selective hydrogenation of m-and p-chloronitrobenzene over PVP-stabilized platinum colloidal catalysts, J. Mol. Catal. A: Chem. 159 (2000) 115-120. |
| [15] | J. Zhang, Y. Wang, H. Ji, et al., Magnetic nanocomposite catalysts with high activity and selectivity for selective hydrogenation of ortho-chloronitrobenzene, J. Catal. 229 (2005) 114-118. |
| [16] | X.X. Han, R.X. Zhou, X.X. Zheng, et al., Effect of rare earths on the hydrogenation properties of p-chloronitrobenzene over polymer-anchored platinum catalysts, J. Mol. Catal. A: Chem. 193 (2003) 103-108. |
| [17] | X.X. Han, R.X. Zhou, G.M. Lai, et al., Effect of transition metal (Cr, Mn, Fe, Co, Ni and Cu) on the hydrogenation properties of chloronitrobenzene over Pt/TiO2 catalysts, J. Mol. Catal. A: Chem. 209 (2004) 83-87. |
| [18] | C. Su, X.N. Li, Q.F. Zhang, et al., Behavior of adsorbed diphenyl-sulfide on the Pd/C catalyst for o-chloronitrobenzene hydrogenation, Chin. Chem. Lett. 24 (2013) 59-62. |
| [19] | B. Coq, A. Tijani, F. Figuéras, Influence of alloying platinum for the hydrogenation of p-chloronitrobenzene over PtM/Al2O3 catalysts with M=Sn, Pb, Ge, Al, Zn, J. Mol. Catal. 71 (1992) 317-333. |
| [20] | B. Coq, A. Tijani, R. Dutartre, et al., Influence of support and metallic precursor on the hydrogenation of p-chloronitrobenzene over supported platinum catalysts, J. Mol. Catal. 79 (1993) 253-264. |
| [21] | B. Zuo, Y. Wang, Q. Wang, et al., An efficient ruthenium catalyst for selective hydrogenation of ortho-chloronitrobenzene prepared via assembling ruthenium and tin oxide nanoparticles, J. Catal. 222 (2004) 493-498. |
| [22] | D. Kashchiev, G. Van Rosmalen, Review: nucleation in solutions revisited, Cryst. Res. Technol. 38 (2003) 555-574. |
| [23] | Y.X. Liu, J.X. Chen, J.Y. Zhang, Effects of the supports on activity of supported nickel catalysts for hydrogenation of m-dinitrobenzene to m-phenylenediamine, Chin. J. Chem. Eng. 15 (2007) 63-67. |
| [24] | E. Esmaeili, Y. Mortazavi, A.A. Khodadadi, A.M. Rashidi, M. Rashidzadeh, The role of tin-promoted Pd/MWNTs via the management of carbonaceous species in selective hydrogenation of high concentration acetylene, Appl. Surf. Sci. 263 (2012) 513-522. |
| [25] | M. Studer, S. Neto, H.U. Blaser, Modulating the hydroxylamine accumulation in the hydrogenation of substituted nitroarenes using vanadium-promoted RNi catalysts, Top. Catal. 13 (2000) 205-212. |
| [26] | F. Cárdenas-Lizana, S. Gómez-Quero, M.A. Keane, Clean production of chloroanilines by selective gas phase hydrogenation over supported Ni catalysts, Appl. Catal. A 334 (2008) 199-206. |
| [27] | X. Meng, H. Cheng, Y. Akiyama, et al., Selective hydrogenation of nitrobenzene to aniline in dense phase carbon dioxide over Ni/g-Al2O3: significance of molecular interactions, J. Catal. 264 (2009) 1-10. |
| [28] | X. Meng, H. Cheng, S.I. Fujita, et al., Selective hydrogenation of chloronitrobenzene to chloroaniline in supercritical carbon dioxide over Ni/TiO2: significance of molecular interactions, J. Catal. 269 (2010) 131-139. |
| [29] | F.Y. Zhao, Y. Ikushima, M. Arai, Hydrogenation of nitrobenzene with supported platinum catalysts in supercritical carbon dioxide: effects of pressure, solvent, and metal particle size, J. Catal. 224 (2004) 479-483. |
| [30] | D. Amalric-Popescu, F. Bozon-Verduraz, Infrared studies on SnO2 and Pd/SnO2, Catal. Today 70 (2001) 139-154. |