b College of Chemistry, Nankai University, Tianjin 300071, China
As petrochemical resources deplete in an alarming rate, producing fuel and other high-value chemicals from renewable biomass has generated sustained interest worldwide. It is an important and challenging path for biomass utilization using selective catalytic routes to process the carbohydrate fractions of lignocellulose to produce valuable platform chemicals [1]. Furfural, derived from lignocellulosic biomass,is a very versatile and key bio-based platform chemical used for the production of important non-petroleum-derived chemicals and the development of new generation of bioplastics [2]. Besides,it is a biofuel precursor and also in high demand in industries including plastics,petroleum refining,agrochemical and pharmaceutical industries [3]. Industrial process for furfural productionviamineral acid treatment of hemicellulose and high pressure saturated steam stripping of furfural suffered from disadvantages such as low efficiency,high energy consumption,equipment corrosion,severe yield loss due to side-reactions and the generation of acidic effluent,etc. Alternative approaches for furfural production investigated recently focused on the development of a variety of different catalysts [4, 5] and reaction medium [6, 7]. Besides,systems using simultaneous stripping (e.g.,N2stripping [8]) or extraction (e.g.,water/organic solvent biphasic systems [9, 10, 11]) for furfural have also been proposed. All the above-mentioned approaches have improved furfural yield and selectivity. However,the production of furfural with minimal carbon footprint route is still a challenging undertaking. Water is definitely a cheap and green solvent for furfural formation. However,the use of water as reaction medium suffers from disadvantages such as low furfural yields and the generation of a large amount of acidic effluent. Recently,due to the negligible vapour pressure,ionic liquids have been widely considered as ‘‘green’’ solvents instead of water or other organic solvents for many reactions including furfural production [7, 12, 13]. Besides,other unique properties of ionic liquid such as high thermal stability,non-flammability,and good solvent power also make ionic-liquid-phase reactions useful for producing biofuels and biochemicals. However,the inherent toxicity of commonly used ILs has not been investigated thoroughly. More importantly,the application of commonly used ionic liquids on industrial scale is limited,owing to their high costs. More recently, deep eutectic solvents (DES) have drawn increasing attention and are revealed to be a promising alternative to ILs as green media [14, 15]. DESs are mixtures of two or three compounds that are capable of forming eutectic liquids,in which choline chloride (ChCl) is a commonly used component [16]. Deep eutectic solvents share many characteristics with ILs and have added advantages of low price,low toxicity,bio-degradability,environmental friendliness,ease to prepare on large scale and the elimination of the preliminary purification step. ChCl-based DESs could be obtained by mixing choline chloride with substances having hydrogen bond donors (urea,carboxylic acids,alcohols,etc.). In this manuscript, ChCl-oxalic acid,characterized by low toxicological risks,low pollution,reduced mobility,bulk renewable and acidic property, was selected as an alternative media for ‘‘greener’’ furfural production processes.
As for the catalysts employed in the production of furfural,both Brønsted acid and Lewis acid catalysts catalyze the reactions in furfural formation [17]. Recently,metal chloride as catalyst [12, 18, 19] or additive [20, 21] for the conversion of lignocelluloses into furfural have been reported. Metal chloride clearly accelerated the reaction rate of the furfural formation from xylose and it was found that both metal cation and Cl-ion are responsible for this reaction [20, 22]. Additionally,although it had been established that metal chlorides had pronounced influence on xylose isomerization,the presence of Brønsted acid was recently found to facilitate both the dehydration reactions and the furfural selectivity [20]. Thus the combination of a metal chloride and an acid would probably achieve better furfural yields. The ChCl-oxalic acid mixture could play the role of both reaction medium and Brønsted acid catalyst,and it is also a Cl-ion provider. In addition, industrial processes for furfural production are commonly carried out at temperatures>423 K. If the reactions could be conducted at lower temperatures,energy saving could be substantial. Based on all the reasons above,and with the aim to produce furfural in a milder and more environmentally friendly manner,metal chlorides enhanced production of furfural in ChCl-oxalic acid under mild conditions were investigated in this work. Moreover,one approach to inhibit the formation of humins and thus promote furfural yield and selectivity is to extract furfural simultaneously using an organic solvent as extractant (low boiling solvent is energetically more advantageous,such as MIBK). Therefore reactions in a DES/MIBK biphasic system was also studied in this work to selectively extract furfural from the DES phase to organic phase in order to further enhance the furfural yield. 2. Experimental 2.1. Materials
Xylan (from birch wood,.90%) was purchased from Sigma- Aldrich Ltd.D-Xylose (.98%) and choline chloride (.99%) were purchased from Acros. CrCl3×6H2O,FeCl3×6H2O,AlCl3×6H2O, CeCl3×7H2O,LaCl3×7H2O and oxalic acid were purchased from Tianjin Jiangtian Chemical Co.,Ltd. (Tianjin,China). All other chemicals were purchased from Sigma-Aldrich and used without further purification. ChCl-oxalic acid was synthesized according to procedures reported in the literature [23, 24]. The molar ratio of choline chloride to oxalic acid was following the report by Huet al. [24]. 2.2. Procedure for the reaction in the monophase system
Substrate sample (0.25 mmol),ChCl-oxalic acid (6 mmol based on the acid) and a known amount of metal chloride were loaded into a sealed glass reactor (10 mL). The mixture was then heated in a preheated oil bath and stirred at different temperatures for the desired time. When using xylan as the starting material,10 mg of H2O was added into each reactor. After the desired residence time, the reaction was quenched by putting the reactor in cooling water immediately. Samples were then diluted,filtered and then analyzed using HPLC. Reactions heated by microwave irradiation were conducted according to the procedures reported in our previous work [12]. 2.3. Procedure for the reaction in the DES/MIBK biphasic system
Experiments were carried out in 10 mL sealed thick-walled glass vessels in a preheated oil bath with magnetic stirring. In a typical experiment,the reactor was charged with required amounts of feedstock,metal chloride,ChCl-oxalic acid and MIBK, these materials were then mixed using a magnetic stirrer and heated to the desired temperature and time. After reaction,the reactor was cooled to room temperature by cooling water immediately. The DES phase and organic phase were separated and collected for analysis. 2.4. Quantification procedure for furfural and xylose
Furfural was determined using HPLC (Agilent 1200) with an Ultraviolet Detector and a XDB-C18 column at 280 nm. The column oven temperature was maintained at 303 K. The mobile phase was acetonitrile/water (15/85,v/v) at a flow rate of 1 mL/min.
Quantitative analysis of xylose was performed using HPLC (Waters 1525) equipped with a refractive index detector (Waters 2412) and an aminex HPX-87H column. The column oven temperature was maintained at 338 K. H2SO4(5 mmol/L) was used as the mobile phase at a flow rate of 0.6 mL/min. For HPLC analysis,the samples were filtered with a syringe filter (0.2mm) prior to analysis. Conversion of xylose and yields of products were defined as follows:
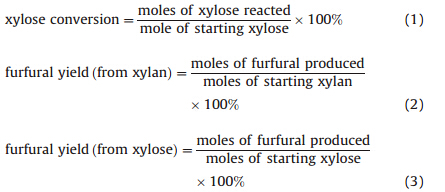
We started our studies by investigating the effect of trivalent metal chlorides in ChCl-oxalic acid in converting xylose into furfural at 373 K. CrCl3×6H2O,FeCl3×6H2O,AlCl3×6H2O,CeCl3×7H2O and LaCl3×7H2O were selected as a co-catalyst. As shown in Fig. 1, furfural was formed in ChCl-oxalic acid without an additional metal chloride affording a furfural yield of 14.6%,indicating that DES acted as both a Brønsted acid catalyst and a reaction media. The furfural yields could be improved by combining a metal chloride as the co-catalyst. Amongst all the trivalent metal chlorides tested,AlCl3×6H2O proved most efficient in producing furfural from xylose,with a 32.4% furfural yield achieved at 74.3% xylose conversion at 373 K in 30 min. For other trivalent metal chlorides,the furfural yields obtained under the typical reaction conditions followed the order of FeCl3×6H2O>CrCl3×6H2O> CeCl3×6H2O>LaCl3×7H2O,leading to furfural yields and xylose conversions in the range of 16%-23% and 61%-69%,respectively.
The mechanism of furfural formation from xylose is still debated and has not been unequivocally established. Specifically, furfural production mechanism may differ when the reaction was catalyzed by different catalysts under different reaction conditions. In this manuscript,a double catalytic effect was achieved by adding metal chlorides to ChCl-oxalic acid because both ChCl- oxalic acid and metal chloride acted as catalyst in the production of furfural from xylose (Scheme 1). It was known that metal chlorides promote the enolization of xylose [25],and the presence of metal chlorides induced xylose dehydration to form furfural via the xylose isomerization to xylulose [10, 26, 27],which is much different from the cases using individual Brønsted acid catalyst. Metal cation could promote the conversion of xylose to furfural by bonding with an oxygen atom. In this work,the furfural yields obtained by different metal chlorides varied when these metal chlorides were added in equal molar dosage,mainly because metal cations catalyze the reaction of carbohydrates into furfural proportionally to their ionization potential [22].
|
Download:
|
| Fig. 1. Conversion of xylose into furfural in ChCl–oxalic acid in the addition of metal chlorides. Reaction conditions: 6 mmol ChCl–oxalic acid, 0.25 mmol xylose, 0.125 mmol metal chlorides, 373 K, 30 min. | |
AlCl3×6H2O produced superior yields in the preliminary tests and hence was selected for further assessments. The effects of temperature,residence time on the reaction were studied,and the results are shown in Table 1. At 353 K,a furfural yield of 14.1% was obtained in 90 min. However,when water was used as the reaction medium as a control,furfural was not produced (Table 1,entry 2).
| Table 1 Furfural formation from xylose in ChCl–oxalic acid in the addition of AlCl3.6H2O.a |
The results indicated that the use of ChCl-oxalic acid as reaction media improved the dehydration of xylose to furfural at 353 K. One reason for this observation could be the acidity of the ChCl-oxalic acid system while another possibility could be that nonaqueous media was beneficial furfural formation [28, 29]. Further increase of the reaction time at 353 K gave higher furfural yield (Table 1,entry 3). Additionally,microwave irradiation resulted in improved results (68% xylose conversion,30.3% furfural yield in 1 h at 353 K) due to this heating method offered a means of rapid, uniform,and selective heating,which resulted in accelerated reaction rates [30, 31]. Compared with traditional heating methods, microwave method minimizes temperature gradients and has the advantage of higher yields in a short reaction time. However,the application of microwave irradiation on industrial scale remained difficult,thus the subsequent experiments were conducted by conventional heating. The yields of furfural improved when the temperature was increased from 353 K to 373 K (furfural yields increased from 14% to 44%,with shorter residence time),indicating that temperature influenced the reaction significantly,and higher temperature favored furfural formation [32]. In addition,further increasing AlCl3×6H2O loading to 0.25 mmol at 373 K,the furfural yield decreased (Table 1,entry 11) with increased solid formation. This trend could be due to the fact that more AlCl3×6H2O accelerated the formation of furfural but also promoted other side reactions (mainly furfural degradation reactions such as fragmentation of furfural,condensation reactions between furfural and xylose or other intermediates,and resinification of furfural) resulted in undesired by-products.
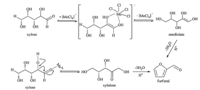
|
Download:
|
| Scheme 1. Putative mechanism for the conversion of xylose into furfural catalyzed by both metal chloride and ChCl–oxalic acid. Me3+ represent trivalent metal cations. | |
Consequently,to further expand the range of feedstock for furfural production in ChCl-oxalic acid with the addition of metal chlorides,the conversion of xylan into furfural was investigated. Specifically,the effect of FeCl3×6H2O was also compared to that of AlCl3×6H2O. The transformation of xylan to furfural was found unappreciable at<373 K in aqueous media before. Binderet al.[26] reported a moderate furfural yield from xylose with acid and chromium catalysts and halide additives at 373 K in DMA. However,when they used similar conditions to convert xylan into furfural,the yields were very low (1%). In this manuscript,it is worth noting that moderate furfural yields were achieved from xylan at 373 K (Table 2). This result indicated the advantage of ChCl-oxalic acid in terms of xylan (or drawing an analogy to the xylose polymers) conversion into furfural at relatively low temperature (e.g.,373 K). As shown in Table 2,without metal chlorides,a furfural yield of 13.3% from xylan was obtained due to the acidic characteristics of ChCl-oxalic acid. FeCl3×6H2O achieved a comparable furfural yield (38.4%) to that of AlCl3×6H2O (39.8%) at equal molar dosage,but with longer residence time (Table 2, entries 2 and 4). The catalytic effect of metal cation is of great interest and worth studying in the future. Furfural yield decreased from 39.8% to 30.1% when the reaction time further prolonged from 50 min to 70 min in the presence of AlCl3×6H2O (Table 2, entries 2 and 5),probably due to the formation of undesired compounds at longer residence time. Increasing AlCl3×6H2O dosage to 0.25 mmol did not result in an increased furfural yield under the typical reaction conditions (Table 2,entries 2 and 6),mainly due to side reactions were accelerated by the excess AlCl3×6H2O.
| Table 2 Conversion of xylan into furfural in the addition of metal chlorides in ChCl–oxalic acid.a |

|
Download:
|
| Fig. 2. Furfural yield in MIBK in the recycle of ChCl–oxalic acid and AlCl3.6H2O. The condition was the same as that of entry 1 in Table 3, under the typical reaction condition, xylose conversion>99%. | |
Subsequently,we concentrated on how to promote furfural yield. In order to design a process that provides furfural in higher yields,side reactions would have to be suppressed. It has been well studied that biphasic systems facilitated the continuous product removal by extracting furfural to the organic phase once it is produced [33] and thus reduced the side reactions and increased the furfural yield and selectivity. Given all this,biphasic systems also offered the possibility of a higher initial feedstock loading. So the biphasic system (DES/MIBK) was investigated in this work for furfural formation from xylose and xylan by increasing the initial substrate loading to 0.5 mmol. As expected (Table 3),simultaneous furfural extraction by MIBK significantly increased the furfural yields in all cases and the highest furfural yield of 60.4% from xylose was achieved at 373 K for 60 min. Moreover,in the biphasic system,ChCl-oxalic acid is not extractable by MIBK and ChCl- oxalic acid and AlCl3×6H2O could be reused after separating the MIBK phase from the reactor and further extracting the product in the DES using fresh MIBK (3 mL×3). After extraction,AlCl3×6H2O was still in the EDS phase. Before reuse,the DES-AlCl3×6H2O was dried at 353 K in a vacuum drier to remove water. Subsequently, the DES-AlCl3×6H2O phase was reused by adding new substrate (0.5 mmol xylose) into the reactor directly. The results of recycling the DES and AlCl3×6H2O (Fig. 2) indicated that the ChCl-oxalic acid and AlCl3×6H2O remained active when they were reused over five consecutive cycles.
| Table 3 Furfural formation in DES/MIBK biphasic system.a |
In summary,both monophase (DES) and biphase (DES/MIBK) routes for processing xylose/xylan to produce furfural were developed in the presence of trivalent metal chlorides in ChCl- oxalic acid. In the monophase reactions,the addition of metal chlorides (especially AlCl3×6H2O) in ChCl-oxalic acid resulted in improved furfural yields and moderate furfural yields (14%-44%) were obtained under very mild conditions (353-373 K). A processing strategy of biphasic system (DES/MIBK) was also studied,and furfural yields obtained from xylose and xylan in the presence of AlCl3×6H2O were 60.4% and 55.5%,respectively. The DES/AlCl3×6H2O phase could be reused to maintain a stable catalytic effect for five successive runs in the biphasic system. However,ChCl-oxalic acid was less stable above 379 K in this catalytic process,which appeared to be the key limiting issue of this approach. The development of acidic green DES with better thermostability should be emphasized in the future work. Acknowledgment
The authors gratefully acknowledge the Major National Science & Technology Projects of China on Water Pollution Control and Treatment (No. 2012ZX07501002-001) for the financial support.
| [1] | F. Geilen, B. Engendahl, A. Harwardt, et al., Selective and flexible transformation of biomass-derived platform chemicals by a multifunctional catalytic system, Angew. Chem. 122 (2010) 5642-5646. |
| [2] | A.S. Mamman, J.M. Lee, Y.C. Kim, et al., Furfural: hemicellulose/xylosederived biochemical, Biofuels Bioprod. Biorefin. 2 (2008) 438-454. |
| [3] | S. Dutta, S. De, B. Saha, M.I. Alam, Advances in conversion of hemicellulosic biomass to furfural and upgrading to biofuels, Catal. Sci. Technol. 2 (2012) 2025-2036. |
| [4] | P.L. Dhepe, R. Sahu, A solid-acid-based process for the conversion of hemicellulose, Green Chem. 12 (2010) 2153-2156. |
| [5] | R. Rinaldi, F. Schüth, Design of solid catalysts for the conversion of biomass, Energy Environ. Sci. 2 (2009) 610-626. |
| [6] | E.I. Gürbüz, J.M.R. Gallo, D.M. Alonso, et al., Conversion of hemicellulose into furfural using solid acid catalysts in γ-valerolactone, Angew. Chem. Int. Ed. 52 (2013) 1270-1274. |
| [7] | C. Sievers, I. Musin, T. Marzialetti, et al., Acid-catalyzed conversion of sugars and furfurals in an ionic-liquid phase, ChemSusChem 2 (2009) 665-671. |
| [8] | I. Agirrezabal-Telleria, J. Requies, M.B. Güemez, P.L. Arias, Furfural production from xylose + glucose feedings and simultaneous N2-stripping, Green Chem. 14 (2012) 3132-3140. |
| [9] | E.I. Gürbüz, S.G. Wettstein, J.A. Dumesic, Conversion of hemicellulose to furfural and levulinic acid using biphasic reactors with alkylphenol solvents, Chem-SusChem 5 (2012) 383-387. |
| [10] | Y. Yang, C.W. Hu, M.M. Abu-Omar, Synthesis of furfural from xylose, xylan, and biomass using AlCl3·6H2O in biphasic media via xylose isomerization to xylulose, ChemSusChem 5 (2012) 405-410. |
| [11] | R. Xing, W. Qi, G.W. Huber, Production of furfural and carboxylic acids from waste aqueous hemicellulose solutions from the pulp and paper and cellulosic ethanol industries, Energy Environ. Sci. 4 (2011) 2193-2205. |
| [12] | L.X. Zhang, H.B. Yu, P. Wang, H. Dong, X.H. Peng, Conversion of xylan, D-xylose and lignocellulosic biomass into furfural using AlCl3 as catalyst in ionic liquid, Bioresour. Technol. 130 (2013) 110-116. |
| [13] | L.X. Zhang, H.B. Yu, P. Wang, Solid acids as catalysts for the conversion of D-xylose, xylan and lignocellulosics into furfural in ionic liquid, Bioresour. Technol. 136 (2013) 515-521. |
| [14] | D.Z. Yang, M.Q. Hou, H. Ning, et al., Efficient SO2 absorption by renewable choline chloride-glycerol deep eutectic solvents, Green Chem. 15 (2013) 2261-2265. |
| [15] | E. Durand, J. Lecomte, B. Baréa, et al., Evaluation of deep eutectic solvent-water binary mixtures for lipase-catalyzed lipophilization of phenolic acids, Green Chem. 15 (2013) 2275-2282. |
| [16] | S. Handy, K. Lavender, Organic synthesis in deep eutectic solvents: Paal-Knorr reactions, Tetrahedron Lett. 54 (2013) 4377-4379. |
| [17] | V. Choudhary, S. Sandler, D. Vlachos, Conversion of xylose to furfural using lewis and Brønsted acid catalysts in aqueous media, ACS Catal. 2 (2012) 2022-2028. |
| [18] | L.Y. Mao, L. Zhang, N.B. Gao, A.M. Li, FeCl3 and acetic acid co-catalyzed hydrolysis of corncob for improving furfural production and lignin removal from residue, Bioresour. Technol. 123 (2012) 324-331. |
| [19] | Y. Yang, C.W. Hu, M.M. Abu-Omar, Conversion of carbohydrates and lignocellulosic biomass into 5-hydroxymethylfurfural using AlCl3 ·6H2O catalyst in a biphasic solvent system, Green Chem. 14 (2012) 509-513. |
| [20] | G. Marcotullio, W. de Jong, Chloride ions enhance furfural formation from Dxylose in dilute aqueous acidic solutions, Green Chem. 12 (2010) 1739-1746. |
| [21] | L. Mao, L. Zhang, N. Gao, et al., Seawater-based furfural production via corncob hydrolysis catalyzed by FeCl3 in acetic acid steam, Green Chem. 15 (2013) 727-737. |
| [22] | J. Gravitis, N. Vedernikov, J. Zandersons, A. Kokorevics, Furfural and levoglucosan production from deciduous wood and agricultural wastes, ACS Symp. Ser. 784 (2001) 110-122. |
| [23] | A.P. Abbott, D. Boothby, G. Capper, et al., Deep eutectic solvents formed between choline chloride and carboxylic acids: versatile alternatives to ionic liquids, J. Am. Chem. Soc. 126 (2004) 9142-9147. |
| [24] | S. Hu, Z. Zhang, Y. Zhou, et al., Direct conversion of inulin to 5-hydroxymethylfurfural in biorenewable ionic liquids, Green Chem. 11 (2009) 873-877. |
| [25] | G. Marcotullio, W. de Jong, Furfural formation from D-xylose: the use of different halides in dilute aqueous acidic solutions allows for exceptionally high yields, Carbohydr. Res. 346 (2011) 1291-1293. |
| [26] | J.B. Binder, J.J. Blank, A.V. Cefali, R.T. Raines, Synthesis of furfural from xylose and xylan, ChemSusChem 3 (2010) 1268-1272. |
| [27] | T. vom Stein, P.M. Grande, W. Leitner, P.D. de María, Iron-catalyzed furfural production in biobased biphasic systems: from pure sugars to direct use of crude xylose effluents as feedstock, ChemSusChem 4 (2011) 1592-1594. |
| [28] | A. Takagaki, M. Ohara, S. Nishimura, et al., One-pot formation of furfural from xylose via isomerization and successive dehydration reactions over heterogeneous acid and base catalysts, Chem. Lett. 39 (2010) 838-840. |
| [29] | J.P. Lange, E. van der Heide, J. van Buijtenen, et al., Furfural-a promising platform for lignocellulosic biofuels, ChemSusChem 5 (2012) 150-166. |
| [30] | R. Weingarten, J. Cho, J.W.C. Conner, G.W. Huber, Kinetics of furfural production by dehydration of xylose in a biphasic reactor with microwave heating, Green Chem. 12 (2010) 1423-1429. |
| [31] | O. Yemiş, G. Mazza, Acid-catalyzed conversion of xylose, xylan and straw into furfural by microwave-assisted reaction, Bioresour. Technol. 102 (2011) 7371-7378. |
| [32] | R. O'Neill, M.N. Ahmad, L. Vanoye, F. Aiouache, Kinetics of aqueous phase dehydration of xylose into furfural catalyzed by ZSM-5 zeolite, Ind. Eng. Chem. Res. 48 (2009) 4300-4306. |
| [33] | J.H. Zhang, J.P. Zhuang, L. Lin, S.J. Lin, Z. Zhang, Conversion of D-xylose into furfural with mesoporous molecular sieve MCM-41 as catalyst and butanol as the extraction phase, Biomass Bioenergy 39 (2012) 73-77. |