b Key Laboratory of Advanced Textile Materials and Manufacturing Technology, Ministry of Education of China, Hangzhou 310018, China;
c Zhejiang Jihua Group Co., Ltd., Hangzhou 311228, China
Disperse dyes with little water solubility are largely produced and widely used for coloration of hydrophobic fibers,such as polyester. Since disperse dyes have limited solubility in water, some dye particles may remain on the fiber surface after the dyeing is completed and cause reduced color fastness on washing and rubbing [1, 2]. Therefore,it is customary to remove the insoluble dye particles by some clearing processes after dyeing. The commonly used method for polyester fabrics is reduction clearing, namely,the dyed fabrics were treated by a solution containing 2 g/L sodium hydrosulfite (as reductant) and 1 g/L sodium hydroxide at 70 ℃ for 15 min [3],which decomposes dispersed dye particles into water soluble small molecules. However,the reduction clearing process causes serious water pollution because of the addition of chemicals and increases the treatment difficulty of the effluents [4]. Additionally,disperse dyes containing azo groups can also be cleaved to aromatic amines and some of them might be toxic and carcinogenic. Therefore,elimination of treatment of reduction clearing in the dyeing polyester process with disperse dyes can significantly reduce the costs of effluent treatments. Encouragingly,alkali-clearable disperse dyes offer a means of resolving the problem [5].
Alkali-clearable dyes,which can be solubilized by the solo action of alkali,have been developed in recent years. Some phthalimide and sulfonyl fluoride containing disperse dyes have been reported due to their alkali-clearable property [6]. Phthalimide containing,alkali-clearable disperse dyes undergo ring opening and convert to water soluble dyes under relatively mild alkaline conditions. As for sulfonyl fluoride containing,alkaliclearable disperse dyes,the incorporated fluorosulfonyl group of these dyes can also be converted to the water soluble dyes containing sulfonate groups under mild alkaline conditions. These water soluble dyes generated after alkali clearing are readily washed off without generation of harmful primary aromatic amines,even if there are some azo groups in their chromophores.
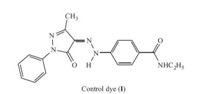
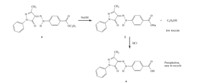
In view of the lower toxicity of the hydrolysate of carboxylic ester group than sulfonyl fluoride and phthalimide groups under alkaline conditions,in this work,a yellow,azo disperse dye containing a carboxylic ester moiety was readily synthesized (see Scheme 1) and its structure was confirmed. The synthesized, ester-containing dye 4 and a similar control dye (I,Fig. 1) containing acylamide moiety [7] were then applied to dyeing poly(ethylene terephthalate) (PET) fabric and their wash and rubbing fastness properties with different after-treatment methods (reduction clearing and alkali clearing) were examined and compared.
|
Download:
|
| Fig. 1.Facile synthetic route of an alkali-clearable azo disperse dye containing the carboxylic ester moiety. | |
|
Download:
|
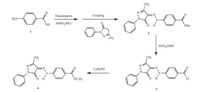
| Scheme 1.The structure of the control dye containing the acylamide moiety. | |
All reagents were of analytic grade and obtained from commercial suppliers and used without further purification. Melting points were measured on a Mel-Temp capillary melting point apparatus and were uncorrected. 1H NMR and 13C NMR spectra were recorded on a Varian INOVA 400 NMR Spectrometer with TMS as internal standard in CDCl3. IR spectra were measured with an FT/IR-430 spectrophotometer. Mass spectra (MS) were determined by using a HP1100 mass spectrometer. Ultraviolet- visible (UV-vis) absorption spectra were recorded on a Lambda 900 UV/vis spectrophotometer.
The synthetic route of the target ester-containing dye is outlined in Scheme 1 which mainly contains three steps as follows.
Synthesis of acid dye 2 by diazo coupling reaction: Acid dye 2 was synthesized by conventional diazo-coupling methods. Initially, p-aminobenzoic acid (1.37 g,10 mmol) was dissolved in a 5% sodium hydroxide aqueous solution (10 mL,10 mmol) at room temperature and a 12% sodium nitrite (6 mL,11 mmol) aqueous solution was rapidly mixed with the amine solution. Subsequently, the amine-nitrite mixture was cooled to 0-5 ℃ and then rapidly poured into the ice-cooled dilute hydrochloric acid (13 mL, 2.3 mol/L) solution already in the vessel. The coupling reaction was carried out by adding the prepared diazonium salt solution to the coupling component (3-methyl-1-phenyl-2-pyrazoline-5-one) solution (1.76 g,10.1 mmol) at 0-5 ℃,pH 8.5-9.0 for 3 h. The pH value of the coupling liquor was controlled by adding sodium carbonate powder. The dye was salted out by adding 2.0 g sodium chloride. Crude yield: 4.0 g (116.3%); FTIR (KBr,cm-1): 2850 (CH3), 1656 (C55O,pyrazolone),1537,1366 (C55O,COONa); MS (ESI, negative): m/z 321.1 [M-Na]-. The crude dye 2 was then purified by the N,N-dimethylformamide/ether (3:20,v/v) purification method [8]. Yield: 1.9 g (55.2%).
Synthesis of acyl chloride 3: N,N-dimethylformamide (0.2 mL) was added to a stirred suspended mixture of acid dye 2 (3.5 g, 10 mmol) and thionyl chloride (20 mL,270 mmol) at room temperature. The mixture was heated to 60 ℃ and stirred for 1.5 h. The solvent thionyl chloride was removed by vacuum distillation,and then the residue was transferred into ice water (1:2 (w/w),150 mL). After it was cooled to room temperature,the product was collected by filtration. The filter was washed with icecooled water until the filtrate was colorless and neutral,then dried in a vacuum. The crude product 3 was purified by recrystallization from toluene. Yield: 2.8 g (77.1%); mp 164-165 ℃; FTIR (KBr, cm-1): 1765 (C55O,acyl chloride),1672 (C55O,pyrazolone); 1H NMR (400 MHz,CDCl3): d 13.58 (s,1H,55N-NH),8.18 (d,2H,Ar-H), 7.94 (d,2H,Ar-H),7.49-7.53 (m,2H,Ar-H),7.42-7.47 (m,3H,Ar- H),2.40 (s,3H,N55C-CH3); MS (ESI,negative): m/z 339.1 [M-H]-, 341.1 [M+2-H]-; Element analysis: Found (%): C,59.85,H,3.93,N, 16.28; Calcd. (%): C,59.92,H,3.85,N,16.44.
Synthesis of ester-containing dye 4: To a stirred suspension of acyl chloride 3 (1.7 g,5 mmol) in dry ethanol (30 mL),K2CO3 (0.7 g, 5 mmol) and a few zeolites were added. The reaction mixture was heated to the boiling point and refluxed for 1.5 h,then the solvent was entirely distilled and the residue was poured into 10% dilute hydrochloric acid (100 mL). The product was collected by filtration, the filter cake was washed with water until the filtrate was colorless and neutral,then the ester-containing dye 4 was air dried at room temperature. The pure dye 4 was obtained by recrystallization in ethanol. Yield: 1.5 g (85.7%); mp 143-145 ℃; FTIR (KBr, cm-1): 2975,2923,2900 (CH3,CH2),1707 (C55O,carboxylic ester), 1665 (C55O,pyrazolone); 1H NMR (400 MHz,CDCl3): d 13.25 (s,1H, 55N-NH),8.01 (d,2H,Ar-H),7.90 (d,2H,Ar-H),7.74 (d,2H,Ar-H), 7.47 (t,2H,Ar-H),7.24 (t,1H,Ar-H),4.33 (q,2H,CH2),2.32 (s,3H, N55C-CH3),1.33 (t,3H,CH3); 13C NMR (125 MHz,CDCl3): d 165.8, 157.3,148.6,144.6,137.8,131.3,129.9,128.9,127.1,125.3,118.3, 115.1,61.1,14.4,11.8; MS (ESI,negative): m/z 348.8 [M-H]-; Element analysis: Found (%): C,65.09,H,5.24,N,16.03; Calcd. (%): C,65.13,H,5.18,N,15.99. 3. Results and discussion
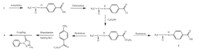
Generally,the commercial,azo disperse dyes are synthesized by a coupling reaction at the last step with the diazo and coupling components prepared in the previous steps (see Scheme 2). The shortcoming of the route focuses on the amino deprotection reaction which probably generates unwanted by-product 5 due to the hydrolysis of the carboxylic ester group,either under acidic or alkaline conditions,and finally fails to yield the target product 4 in high yield.
|
Download:
|
| Scheme 2.Traditional synthetic route of an alkali-clearable azo disperse dye containing the carboxylic ester moiety and its defect. | |
Considering the defect of the traditional synthetic route on synthesis of the ester-containing dye,a facile,synthetic route was designed by avoiding amino protection and deprotection reactions and only proceeds by successive diazotization,coupling reaction, chlorination,and esterification from reactant p-aminobenzoic acid. The detailed synthesis procedure of the ester-containing dye is shown in Scheme 1.
The chemical structure of dye 4 was confirmed by FTIR,1H NMR, 13C NMR,mass spectrometry and elemental analysis. It is known that azopyrazolone dyes predominantly exist in the hydrazone form over the azo formin the solid state and acidic solutions [9]. The FTIR and 1HNMR spectra of the dye provide somecharacteristic results to prove this. The stretching vibration band of carbonyl appears at 1665 cm-1 in the FTIR spectrum. The hydrogen-bonded NH proton appears at d 13.25 in the 1HNMR spectrumand its hydrogen integral is close to 1.0. The results suggest that dye 4 nearly completely exists in the hydrazone form (see Scheme 3).

|
Download:
|
| Scheme 3.Changes between hydrazone and azo forms of ester-containing phenylazopyrazolone dyes 4. | |
In order to prove the alkali-clearing ability of dye 4,dye I has been used for color fastness comparison with different aftertreatment methods. First of all,dye 4 and dye I had been milled for 8 h with pea gravel at room temperature in the presence of dispersing agent NNO (Dye:NNO = 1:1,w/w). Then the dyes were utilized in the dyeing of PET fabric samples in stainless steel sealed dye pots in an infrared high temperature (130 ℃) dyeing machine, using a liquor ratio of 50:1. In the next step,reduction clearing (1 g/L NaOH and 2 g/L Na2S2O4,liquor-to-goods ratio 80:1,at 70 ℃,for 15 min) and alkali clearing (1 g/L NaOH,liquor-to-goods ratio 80:1, at 70 ℃,for 15 min) were carried out on the dyed samples. After clearing,the sampleswere rinsedwith coldwater and air dried. The color fastness properties of various PET samples were then assessed according to ISO standard [10, 11].
The dye sorptions and K/S values of dye 4 and dye I on PET fabric at 130 ℃ for 1 h are listed in Table 1. It can be seen that both of them show high sorption (>94%) and satisfied K/S values (>14) for color fastness tests at 0.5% owf. Table 1 also shows the washing and rubbing fastness test results of dye 4 and dye I on PET fabrics with various treatment methods. Clearly,the treated samples of dye 4 and dye I by reduction clearing and alkali clearing show superior rubbing fastness and washing fastness for cotton staining in comparison with those of the untreated samples. It confirms the effectiveness of the clearing processes to remove the insoluble dye particles on the fiber surface. For dye 4,the sample treated by alkali clearing shows equal fastness properties with the sample treated by reduction clearing. While for the control dye without carboxylic ester group,the sample treated by alkali clearing shows inferior wet rubbing fastness and wash fastness for cotton staining in comparison with the sample treated by reduction clearing. It means dye 4 has good alkali-clear ability than that of dye I due to its easy hydrolysis and washability. In a word,the reductant-free treatment method could get the same color fastness results and simplify the after-treatment process of dyeing wastewater.
| Table 1 Dyeing and color fastness properties of ester-containing dye 4 and dye I on PET fabrics at 0.5% owf. |
Additionally,ester-containing dye 4 has some other advantages on application and after-treatment of dyeing effluents. The hydrolysates of dye 4,namely acid dye 2 and ethanol,in which ethanol is of low toxic and acid dye 2 can easily be reused by acidification,precipitation,filtration and then recycled (see Scheme 4).
|
Download:
|
| Scheme 4.The hydrolysis of alkali-clearable azo disperse dye containing the carboxylic ester moiety in alkali medium and the recycling process of hydrolyzate dye. | |
An alkali-clearable azo disperse dye containing a carboxylic ester moiety was facilely synthesized from carboxylic-containing azo acid dye as reactant by chlorination and esterification with ethanol in high yield. The defect of the traditional synthetic route of azo dyes,which could generate unwanted by-products,was avoided. The ester-containing,alkali-clearable dye could be applied to PET fabric and attain the same excellent wet color fastnesses after alkali clearing treatment without the utilization of reductants in comparison with that after reduction clearing treatment. The ester-containing,disperse dye shows good alkaliclear ability and results in little contamination to the environment due to the absence of reductants,as well as low toxicity and easy recycling of the hydrolysates. Acknowledgments
The work was supported by the National Natural Science Foundation of China (Nos. 51173168,21106135),Zhejiang Provincial Key Innovation Team (No. 2010R50038),Zhejiang Provincial Top Key Academic Discipline of Chemical Engineering and Technology of Zhejiang Sci-Tech University,and "521" Talent Project of Zhejiang Sci-Tech University.
| [1] | J. Koh, H.Y. Yoo, J.P. Kim, One-bath dyeing of poly(ethylene terephthalate)/cotton blends with alkali-clearable azo disperse dyes containing a fluorosulphonyl group, Color. Technol. 120 (2004) 156-160. |
| [2] | J. Koh, Alkali-hydrolysis kinetics of alkali-clearable azo disperse dyes containing a fluorosulphonyl group and their fastness properties on PET/cotton blends, Dyes Pigm. 64 (2005) 17-23. |
| [3] | Standardization Administration of the People's Republic of China, Disperse dyestuff Determination of shade and relative strength, GB/T 2394-2013. |
| [4] | J.H. Choi, J.Y. Choi, E.M. Kim, et al., Coloration properties and clearability of phthalimide-derived monoazo disperse dyes containing ester groups, Color. Technol. 129 (2013) 352-359. |
| [5] | J.S. Koh, J.P. Kim, Synthesis of phthalimide-based alkali-dischargeable azo disperse dyes and analysis of their alkali-hydrolysis mechanism, Dyes Pigm. 37 (1998) 265-272. |
| [6] | J.S. Koh, J.P. Kim, Application of phthalimide-based alkali-clearable azo disperse dyes on polyester and polyester/cotton blends, J. Soc. Dyer. Colour. 114 (1998) 121-124. |
| [7] | Z.H. Cui, X.D. Wang, W.G. Chen, E.L. Hua, K. Liu, Synthesis, spectral and dyeing properties of phenylazopyrazolone-containing acylamide disperse dyes designed for poly(lactic acid), Color Technol. 128 (2012) 283-289. |
| [8] | J. Yang, Analysis and Anatomy of Dyes, Chemical Industry Press, Beijing, 1989. |
| [9] | K. Hunger, Industrial Dyes: Chemistry, Properties, Applications, Wiley-VCH Verlag GmbH & Co. KgaA, Weinheim, 2003. |
| [10] | ISO 105-C03:1989 Textiles-Tests for colour fastness-Part C03: Colour fastness to washing: Test 3.(ISO, 1989). |
| [11] | ISO 105-X12:1993 Textiles-Tests for colour fastness-Part X12: Colour fastness to rubbing.(ISO, 1993). |