The nitrile functionality is one of the most elementary building blocks in chemistry,and has wide applications in synthetic chemistry and pharceuticals [1]. Conventional methods to construct nitriles involved the cyanation of halides [2, 3],the Sandmeyer reaction [4],dehydration of aryl oximes [5]. However, many of these methods suffer from the drawback of employing harsh reaction conditions,such as high temperature or pressure and large excess of reactants [6]. Therefore,the development of feasible and chemically economical approaches to synthesis of nitriles is highly required. Recently,Jiao and co-workers reported a copper catalyzed conversion of methylarenes to aryl nitriles,and successively,they realized the transformation of benzyl halides to aryl nitriles [7]. More recently,Prabhu and co-workers reported a convenient method to synthesize nitriles from primary azides catalyzed by copper iodide,and then achieved a selective oxidation of aliphatic primary azides to the corresponding nitriles in the presence of KI and TBHP [6, 8].
Electrochemical synthesis,an environmentally friendly process, has attracted much interest of chemists and great efforts have been made to gain excellent achievements in recent years [9]. Compared with the conventional redox process,the notable superiority of electrochemical conversion is a mass-free electron transfer between the electrode and the substrate during reactions,which making this method to be green and sustainable. In this context,we report an efficient and easy-to-handle method to synthesize aryl nitriles via a metal-free anodic oxidation process. A series of aryl nitriles were obtained in moderate to good yields directly from benzylic azides at room temperature. 2. Experimental
1H NMR spectra were recorded on a Bruker AVIII-400 spectrometer. Chemical shifts (in ppm) were referenced to tetramethylsilane in CDCl3 as an internal standard. 13C NMR spectra were obtained by using the same NMR spectrometers and were calibrated with CDCl3 (d 77.00 ppm). The spectral data and spectra of all compounds can be found in Supporting information.
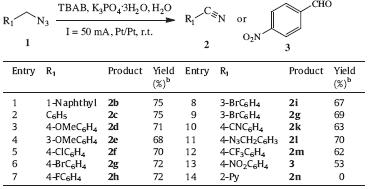
Initial optimization studies were carried out by employing 1- (azidomethyl)naphthalene (1a) as a model substrate. In an electrochemical setup consisting of an undivided cell equipped with a pair of platinum electrodes,a mixture of 1a (0.5 mmol), electrolyte (0.25 mmol),and phrase transfer catalyst (PTC, 0.25 mmol) in different solvents (6 mL) was electrolyzed under galvanostatic conditions and stirred simultaneously for 4 h at room temperature (Table 1). First,the solvent of the reaction was optimized with K3PO4·3H2O as an electrolyte and TBAB as a PTC at a constant current of 20 mA (entries 1 and 2). However,no desired product was obtained. When the current of the reaction system was increased to 50 mA,the desired nitrile was obtained in 73% yield (entry 3). Nevertheless,increasing the current to 80 mA did not show any significant influence in enhancing the yield (entry 4). Subsequently,a series of electrolyte were optimized,and the results showed that K3PO4 was the best electrolyte (entries 3 vs. 5- 7). Finally,other PTCs were tested in this reaction,but only trace of desired nitrile was obtained (entries 8 and 9). Other typical reaction parameters,such as other organic solvent,reaction temperature and concentration of the reactant,were also investigated in this electrochemical transformation; however,no significant improvement in yield was obtained,so we do not discuss the relevant details here.
| Table 1 Optimization of the reaction conditions.a |
Under the optimized reaction condition,a series of substituted azides were investigated to test the generality of this catalytic system. As shown in Table 2,benzylic azides bearing both electrondonating and electron-withdrawing groups transformed into the corresponding nitriles smoothly in moderate to good yields (Table 2,entries 1-12). Interestingly,diazide derivative 1,4- bis(azidomethyl)benzene was oxidized into desired p-cyanobenzene (2l) in good yield (70%,Table 2,entry 11). Unexpectedly,when 1-(azidomethyl)-4-nitrobenzene was employed as the substrate, p-nitrobenzaldehyde 3 was obtained in 53% yield instead of the corresponding nitrile (Table 2,entry 13). This might because azides with strong electron-withdrawing groups undergo a hydrolyzation process more easily during reactions. Heterocyclic substituted azides failed to afford the corresponding products under the typical reaction condition (Table 2,entry 14).
| Table 2 The scope of electrochemical oxidation of azides to nitriles.a |
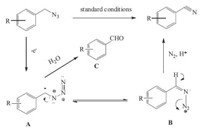
The plausible mechanism of this transformation is presented in Scheme 1. First,the aryl azide is electrochemically oxidized to the benzyl cation A in two steps [10, 11],and then the cation A undergoes Schmidt-type rearrangement [12] to afford the desired product aryl nitrile. During this process,if the intermediates A bearing a strong electron-withdrawing group,A could be attacked by water,resulting in the formation of aldehyde C [13]. This conversion is similar to Jiao and co-workers’ previous research,in which they achieved the transformation from benzyl halides to aryl nitriles by using DDQ as the oxidant [7].
|
Download:
|
| Scheme 1.A proposed mechanism for the reaction. | |
In summary,we have developed a new method to synthesize aryl nitriles via a metal-free electrochemical oxidation process directly from primary benzylic azides. To the best of our knowledge,this is the first report of metal-free oxidation of benzylic azides to nitriles under electrochemical conditions. This electrochemical method is environmentally friendly and easy to handle,thus making it more attractive to aryl nitrile synthesis. Acknowledgment
This work was financially supported by the National Natural Science Foundation of China (Nos. 2127222,91213303,21172205, J1030412). Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.04.024.
| [1] | (a) A.J. Fatiadi, Preparation and synthetic applications of cyano compounds, in: S. Patai, Z. Rappoport (Eds.), Triple-Bonded Functional Groups, vol. 2, Wiley, New York, 1983; (b) S. Arseniyadis, K.S. Kyler, D.S. Watt, Addition and substitution reactions of nitrile-stabilized carbanions, in: W.G. Dauben (Ed.), Organic Reactions, Wiley, New York, 1984;(c) R.C. Larock, Comprehensive Organic Transformations, vol. 102, VCH, New York, 1989, pp. 964-965; (d) A. Kleemann, J. Engel, B. Kutscher, D. Reichert, Pharmaceutical Substance, Synthesis, Patents, Applications, 4th ed., Georg Thieme, Stuggart, 2001; (e) J.S. Miller, J.L. Manson, Designer magnets containing cyanides and nitriles, Acc. Chem. Res. 34 (2001) 563-570; (f) M.B. Smith, J. March, March's Advanced Organic Chemistry: Reactions, Mechanisms and Structure, 6th ed., Wiley, Hoboken, NJ, 2007; (g) P. Magnus, D.A. Scott, M.R. Fielding, Direct conversion of α,β-unsaturated nitriles into cyanohydrins using Mn(dpm)3 catalyst, dioxygen and phenylsilane, Tetrahedron Lett. 42 (2001) 4127-4129. |
| [2] | (a) K.W. Rosenmund, E. Struck, Das am Ringkohlenstoff gebundene Halogen und sein Ersatz durch andere Substituenten. I. Mitteilung: Ersatz des Halogens durch die Carboxylgruppe, Ber. Dtsch. Chem. Ges. 2 (1919) 1749-1756; (b) D.F. Mowry, The preparation of nitriles, Chem. Rev. 42 (1948) 189-283; (c) K. Friedrich, K. Wallenfels, The chemistry of the cyano group, in: Z. Rappoport (Ed.), The Chemistry of the Functional Group, Wiley Interscience, New York, 1970, pp. 67-122; (d) J. Lindley, Copper assisted nucleophilic substitution of aryl halogen, Tetrahedron 40 (1984) 1433-1456; (e) P. Kurtz, Houben-Weyl: Methoden der Organischen Chemie, 4th ed., Georg Thieme, Stuttgart, 1952. |
| [3] | (a) T. Schareina, A. Zapf, M. Beller, Potassium hexacyanoferrate(Ⅱ)-a new cyanating agent for the palladium-catalyzed cyanation of aryl halides, Chem. Commun. (2004) 1388-1389; (b) D. Wang, L. Kuang, Z. Li, K. Ding, L-Proline-promoted rosenmund-von braun reaction, Synlett (2008) 69-72; (c) H.J. Cristau, A. Ouali, J.F. Spindler, M. Taillefer, Mild and efficient copper-catalyzed cyanation of aryl iodides and bromides, Chem. Eur. J. 11 (2005) 2483-2492; (d) J. Zanon, A. Klapars, S.L. Buchwald, Copper-catalyzed domino halide exchangecyanation of aryl bromides, J. Am. Chem. Soc. 125 (2003) 2890-2891. |
| [4] | (a) T. Sandmeyer, Ueberführung der drei Nitraniline in die Nitrobenzoësäuren, Ber. Dtsch. Chem. Ges. 18 (1885) 1492-1496; (b) T. Sandmeyer, Ueber die Ersetzung der Amid-gruppe durch Chlor, Brom und Cyan in denaromatischen Substanzen, Ber.Dtsch.Chem.Ges. 17 (1884) 2650-2653. |
| [5] | (a) E. Choi, C. Lee, Y. Na, S. Chang, [RuCl2(p-cymene)]2 on carbon: an efficient, selective, reusable, and environmentally versatile heterogeneous catalyst, Org. Lett. 4 (2002) 2369-2371; (b) K. Yamaguchi, H. Fujiwara, Y. Ogasawara, M. Kotani, N. Mizuno, Inside cover: aerobic oxidation of alcohols at room temperature and atmospheric conditions catalyzed by reusable gold nanoclusters stabilized by the benzene rings of polystyrene derivatives, Angew. Chem. Int. Ed. 46 (2007) 3922-3925. |
| [6] | M. Lamani, K. Prabhu, An efficient oxidation of primary azides catalyzed by copper iodide: a convenient method for the synthesis of nitriles, Angew. Chem. Int. Ed. 49 (2010) 6622-6625. |
| [7] | (a) W. Zhou, L. Zhang, N. Jiao, Direct transformation of methyl arenes to aryl nitriles at room temperature, Angew. Chem. Int. Ed. 48 (2009) 7094-7097; (b) C. Qin, N. Jiao, Iron-facilitated direct oxidative C-H transformation of allylarenes or alkenes to alkenyl nitriles, J. Am. Chem. Soc. 132 (2010) 15893-15895; (c) W. Zhou, J. Xu, L. Zhang, N. Jiao, An efficient transformation from benzyl or allyl halides to aryl and alkenyl nitriles, Org. Lett. 2010 (12) (2010) 2888-2891. |
| [8] | M. Lamani, P. Devadig, K.R. Prabhu, A non-metal catalysed oxidation of primary azides to nitriles atambienttemperature, Org. Biomol. Chem. 10 (2012) 2753-2759. |
| [9] | (a) L. Zhang, H. Chen, Z. Zha, Z.Y. Wang, Electrochemical tandem synthesis of oximes from alcohols using KNO3 as the nitrogen source, Mediated by tin microspheres in aqueous medium, Chem. Commun. 48 (2012) 6574-6576; (b) L. Zhang, J.H. Su, Z. Zha, Z.Y. Wang, Direct electrochemical imidation of aliphatic aminesvia anodic ooxidation, Chem. Commun. 47 (2011) 5488-5490; (c) L. Meng, J.H. Su, Z. Zha, Z.Y. Wang, Direct electrosynthesis of ketones from benzylic methylenes by electrooxidative C-H activation, Chem. Eur. J. 19 (2013) 5542-5545; (d) Z.L. Zhang, J.H. Su, Z. Zha, Z.Y. Wang, A novel approach for the one-pot preparation of α-ketoamides by anodic oxidation, Chem. Commun. 49 (2013) 8982-8984; (e) H.Y. Ma, Z. Zha, Z.Y. Wang, Electrosynthesis of oxadiazoles from benzoylhydrazines, Chin. Chem. Lett. 24 (2013) 780-782. |
| [10] | (a) C.J. Li, Cross-dehydrogenative coupling (CDC): exploring C-C bond formations beyond functional group transformations, Chem. Res. 42 (2009) 280-2891; (b) D.R. Buckle, in: L.A. Paquette (Ed.), Encyclopedia of Reagents for Organic Synthesis, John Wiley & Sons, Chichester, UK, 1995; (c) D. Walker, J.D. Hiebert, 2,3-Dichloro-5,6-dicyanobenzoquinone and its reactions, Chem. Rev. 67 (1967) 153-159; (d) Y.Z. Li, B.J. Li, X.Y. Lu, S. Lin, Z.J. Shi, Cross dehydrogenative arylation (CDA) of a benzylic ch bond with arenes by iron catalysis, Angew. Chem. Int. Ed. 48 (2009) 3817-3820; (e) Y. Zhang, C.J. Li, Highly efficient cross-dehydrogenative-coupling between ethers and active methylene compounds, Angew. Chem. Int. Ed. 45 (2006) 1949-1952. |
| [11] | For selected examples on radical intermediates, see: (a) J.C. Walton, A. Studer, Evolution of functional cyclohexadiene-based synthetic reagents: the importance of becoming aromatic, Acc. Chem. Res. 38 (2005) 794-802; (b) W.P. Liu, Y.M. Li, K.S. Liu, Z.P. Li, Iron-catalyzed carbonylation-peroxidation of alkenes with aldehydes and hydroperoxides, Am. Chem. Soc. 133 (2011) 10756-10759; (c) K. Xu, Y.B. Hu, S. Zhang, Z.G. Zha, Z.Y. Wang, Direct amidation of alcohols with n-substituted formamides under transition-metal-free conditions, Chem. Eur. J. 18 (2012) 9793-9797; (d) K. Xu, Y. Fang, Z.C. Yan, Z.G. Zha, Z.Y. Wang, A highly tunable stereoselective dimerization of methyl ketone: efficient synthesis of E-and Z-1,4-enediones, Org. Lett. 15 (2013) 2148-2151. |
| [12] | (a) S. Lang, J.A. Murphy, Azide rearrangements in electron-deficient systems, Chem. Soc. Rev. 35 (2006) 146-156; (b) M. Sprecher, D. Kost, The Schmidt reaction of dialkyl acylphosphonates, J. Am. Chem. Soc. 116 (1994) 1016-1026; (c) C.E. Katz, J. Aube, Unusual tethering effects in the Schmidt reaction of hydroxyalkyl azides with ketones: cation-π and steric stabilization of a pseudoaxial phenyl group, J. Am. Chem. Soc. 125 (2003) 13948-13949; (d) D.J. Gorin, N.R. Davis, F.D. Toste, Gold(Ⅰ)-catalyzed intramolecular acetylenic Schmidt reaction, J. Am. Chem. Soc. 127 (2005) 11260-11261; (e) L. Yao, J. Aube, Cation-π control of regiochemistry of intramolecular Schmidt reactions en route to bridged bicyclic lactams, J. Am. Chem. Soc. 129 (2007) 2766-2767. |
| [13] | J.P. Richard, T.L. Amyes, Y.G. Lee, V. Jagannadham, Demonstration of the chemical competence of an iminodiazonium ion to serve as the reactive intermediate of a Schmidt reaction, J. Am. Chem. Soc. 116 (1994) 10833-10834. |