b Department of Pharmaceutical Chemistry, Progressive Education Society's Modern College of Pharmacy Nigdi, Pune 411044, India
1. Introduction
Multicomponent reactions (MCRs) have emerged as a valuable tool in the preparation of structurally diverse chemical libraries of heterocyclic compounds [1]. They are inherently atom economical processes in which relatively complex products can be obtained in a one-pot reaction from simple starting materials,and thus they exemplify many of the desired features of an ideal synthesis. MCRs are generally much more environmentally friendly and offer access to large compound libraries with diverse functionalities with the avoidance of protection and deprotection steps for possible combinatorial surveying of structural variations. In view of the increasing interest in the preparation of a large variety of heterocyclic compound libraries,the development of new synthetically valuable MCRs with several diversity points remains a challenge for both academic and industrial institutions [2].
Thiophene and its derivatives are an important class of heterocyclic compounds possessing broad biological activities, such as anti-inflammatory [3],analgesic [3],antioxidant [4], antitubercular [5],antidepressant [6],sedative [6],antiamoebic [7],oral analgesic [8],anti-metabolite [9],and antineoplastic properties [10]. From the aforementioned reports,it seemed that the development of an efficient,rapid,and clean synthetic route towards focused libraries of such compounds is of great importance to both medicinal and synthetic chemists. Hence in this paper,we report a one-pot,three-component reaction for the synthesis of 4-acetyl tetra-substituted thiophene derivatives and antimicrobial evaluation.
All the synthesized compounds were characterized using FT-IR, 1H NMR,13C NMR,and mass spectrometry and were subjected to minimum inhibitory concentration (MIC) antimicrobial screening against two Gram-positive bacteria (Staphylococcus aureus,Bacillus subtilis),two Gram-negative bacteria (Escherichia coli,Pseudomonas aeruginosa),and two fungi (Candida albicans,Aspergillus niger) using the serial plate dilution method.
2. ExperimentalAll chemicals were purchased from Sigma Aldrich,SD Fine, Spectrochem,Merck,and Himedia. Yields refer to purified products and are not optimized. Melting points were determined on a VEEGO-VMP I melting point apparatus and are uncorrected. IR spectra were recorded on a JASCO-FTIR 4100 spectrophotometer. 1H NMR were recorded on aMERCURY VARIAN 300MHz instrument and chemical shifts (δ)were reported in parts permillion (ppm)with CDCl3 (7.26 ppm) as the solvent. TMS was used as the internal standard for NMR. MS analyses were done on an Applied Biosystem API 2000. Thin layer chromatography (TLC) was performed on precoated aluminium plates with silica gel 60 F254.
General procedure for synthesis of compounds 4a-4n: acetyl acetone 1 (1.0 mmol,1 equiv.) and dried potassium carbonate (1.0 mmol,1 equiv.) were added in dimethyl formamide (3 mL), and the mixture was stirred for 1 h at room temperature. Aryl isothiocyanate 2a-2e (1.0 mmol,1 equiv.) was then added drop wise,and the mixture was stirred for 1 h at room temperature. Then,bromoethylpyruvate 3a (1.0 mmol,1 equiv.) or chloroethylacetoacetate 3b (1.0 mmol,1 equiv.) or dichloroacetone 3c (0.5 mmol,0.5 equiv.) was added and the reaction mixture was heated for 1 h. The reaction was quenched with 10 mL water. The crude product 4a-4n precipitated and was purified by filtration followed by crystallization in methanol.
Physical,analytical and spectroscopic characterization data of compounds 4a-4n are given in Supporting information.
3. Results and discussion 3.1. ChemistryWe hypothesized that the N,S-ketene acetal obtained by condensation of acetyl acetone 1 with substituted phenyl isothiocynates 2a-e would react in situ with 2-chloromethyl derivatives 3a-3c in the presence of potassium carbonate to give the target compounds 4a-4n in one step. Our initial investigations were mainly aimed at finding a suitable base and solvent for the one-pot preparation of target compounds 4a-4n.
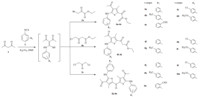
After careful experimentation,we discovered that 1.0 equiv. of acetyl acetone 1,reacts with 1.0 equiv. of phenyl isothiocynates 2a-e in the presence of 1.0 equiv. of potassium carbonate in DMF at room temperature to give N,S-ketene acetals. To this unisolated intermediate,1.0 equiv. of 3a-3b or 0.5 equiv. of 3c was added and the resultant reaction mixture when stirred at room temperature for 1.0 h gave 4a-4n in moderate to good yields (Scheme 1). The main attractions of this protocol are short reaction time and elimination of the intermittent workup procedures necessary to isolate the intermediates,thus directly leading to the formation of the target compounds. Encouraged by the successful results,we synthesized target compounds 4a-4n from the reactions of the acetyl acetone 1,phenyl isothiocynates 2a-2e,and 2-chloromethyl derivatives 3a-3c by using the same strategy and the different substituents.
|
Download:
|
| Scheme 1.The synthesis route for compounds 4a-4n. | |
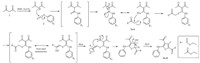
During the reaction,a proton from the active methylene of the acetylacetone 1 gets abstracted by the base added,i.e. K2CO3,to give a nucleophile. This nucleophile then attacks the electron deficient carbon of the phenyl isothiocynates 2a-2e to give the intermediate i.e. N,S-ketene acetals. After the addition of chloromethyl derivatives 3a-3b,the electronegative sulfur of the intermediate adduct attacks the electron deficient carbon atom of chloromethyl derivatives 3a-3b. The keto-enol tautomerism takes place,and then intramolecular cyclisation takes place to give 4- acetyl tetra-substituted thiophene derivatives 4a-4i with the removal of water as shown in Scheme 2. In the case of dichloro acetone 3c the same mechanism takes place at both ends as shown in Scheme 3 to give target compounds 4j-4n.
|
Download:
|
| Scheme 2.The synthesis route for compounds 4a-4n. | |
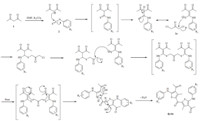
|
Download:
|
| Scheme 3.The synthesis route for compounds 4a-4n. | |
The title compounds 4a-4nwere characterized by 1HNMR,FT-IR, and mass spectra. The IR spectra of title compounds 4a-4e show a peak at 3441.35 cm-1 for an amino group,at 1634.38 cm-1 for a ketone group,at 2990.09 cm-1 for an aromatic group,and at 1726.94 cm-1 for anester group.The 1HNMRspectrumof ethyl2-(4- acetyl-3-methyl-5-(phenylamino)thiophen-2-yl)-2-oxoacetate 4a shows a triplet at δ 1.40 for the 3 protons of a methyl group,singlet for the 3 protons of a methyl group at δ 2.62,a multiplet for the 2 protons of a methylene group at δ 4.42,a multiplet for an aromatic region proton at δ 7.3-7.5,a singlet for a -NH proton at δ 12.04. 13C NMR spectral data of the title compound 4a: The signals at around δ 9.70,13.80 stand for methyl and δ 28.80 stands for methoxy,while signals around δ 117.80-195.60 are attributed to all the aromatic carbons of compound 4a. Also,a distinctive signal at δ 60.80 stands for methylene carbon. Themass spectra of compound 4a show a molecular ion peak at 332.2 and calculated mass of compound found to be 331.09. The IR spectrum of title compounds 4f-4i shows at 3427.85 cm-1 a peak for an amino group,at 1636.3 cm-1 a peak for a ketone group,at 2922.59cm-1 for an aromatic group,and at 1744.3 cm-1 for an ester group. The 1HNMRspectrumfor ethyl 3-(4-acetyl-3-methyl-5-(phenylamino)- thiophen-2-yl)-3-oxopropanoate 4f shows at δ 1.29 a triplet for the 3 protons of a methyl group,at δ 2.6 a singlet for the 3 protons of a methyl group,at δ 2.82 a singlet for the 3 protons of a methyl group,at δ 3.8 a singlet for the 2 protons of a methylene group,at δ 4.25 a multiplet for the 2 protons of a methylene group,at δ 7.3-7.5 a multiplet for aromatic region proton,and at δ 11.96 shows singlet for -NH proton. 13C NMR spectral data of the title compound 4f: The signals at around δ 9.78,14.10 stand for methyl and δ 28.85 stands for methoxy,while signals around δ 117.80-195.60 are attributed to all the aromatic carbon of compound 4f. Also,the distinctive signals δ 49.71 and 60.80 stand for methylene carbon. Mass spectra of compound 4f shows molecular ion peak at 346.3 and calculated mass of compound found to be 345.10. The IR spectra of title compounds 4j-4n show peak at 3436.53 cm-1 for the an amino group,at 1632.45 cm-1 for ketone group,and at 2927.41 cm-1 for aromatic group. The 1H NMR spectrum of di((4-acetyl-3-methyl-5-phenylamino)- thiophen-2-yl)ketone 4j shows at δ 2.58 a singlet for the 6 protons of two methyl groups,at δ 2.6 a singlet for the 6 protons of two methyl groups,at δ 7.2-7.4 a multiplet for an aromatic region proton,and at δ 12.11 a singlet for two -NH protons. 13C NMR spectral data of the title compound 4j: The signal at around δ 9.72 stands for methyl and δ 28.82 stands for methoxy, while signals around δ 117.89-195.60 are attributed to all the aromatic carbons of compound 4j. Mass spectra of compound 4j show a molecular ion peak at 489.3,and the calculated mass of the compound found to be 488.12.
3.2. Antimicrobial studiesAll the synthesized compounds were analyzed for antimicrobial activity. The in vitro antibacterial and antifungal activity of the title compounds was determined by the serial dilution method [11, 12, 13, 14]. The minimum inhibitory concentration (MIC) is given in mg/mL. Samples of different concentrations (0-50 mg/mL) were prepared using dilutions of a 100 mg/mL stock solution,prepared by dissolving the compounds in dimethyl sulfoxide and adjusting the volume to 100 mL. Under identical conditions,Azithromycin and Fluconazole were tested as the reference standard drugs for bacteria and fungi respectively. Synthesized compounds were tested for activity against two Gram-positive bacteria (B. subtilis,S. aureus),two Gram-negative bacteria (P. aeruginosa,E. coli),and two fungal strains (C. albicans,A. niger). Specifications of the microorganisms are given in Table 1. The synthesized series showed excellent to good activity against Gram-negative bacteria (P. aeruginosa and E. coli) and the least activity against Gram-positive bacteria (S. aureus and B. subtilis). All compounds of the series exhibited excellent to moderate antifungal activity against A. niger and C. albicans. Examination of the antimicrobial data (Table 2) revealed that against Gram-positive bacteria S. aureus,compounds 4a,4c,and 4j were found to be equipotent to azithromycin. Compounds 4d-4h and 4k have shown MIC values (1 mg/mL), indicating good antibacterial activity. Against the species B. subtilis, compound 4f shows more potency than standard azithromycin. The compounds 4d and 4e are equipotent to azithromycin. Compounds 4a-c and 4g-k show moderate to good activity. Towards E. coli,compounds 4a-4n shows more potency than the standard. Against P. aeruginosa,compounds 4a-4d and 4f-4m shows more activity than standard azithromycin. All compounds show moderate to minimal activity against fungi C. albicans and A. niger.
| Table 1 Specification of microorganisms. |
| Table 2 Minimum inhibitory concentration. |
The structure-activity relationship study (SAR) indicates that a change in the substituent might also affect the antibacterial activity of title compounds 4a-4n. Compounds having R=H/Cl appeared to have more potential against Gram-positive bacteria S. aureus,Gram-negative bacteria E. coli and P. aeruginosa; and moderate potential against fungal pathogens C. albicans and A. niger. Compounds having R=CH3/OCH3 were found to be more active against Gram-positive B. subtilis,Gram-negative bacteria E. coli,and P. aeruginosa.
4. ConclusionWe have reported a one-pot synthesis of ethyl 2-(4-acetyl-3- methyl-5-(phenylamino)thiophen-2-yl)-2-oxoacetate derivatives 4a-4e,ethyl 3-(4-acetyl-3-methyl-5-(phenylamino)thiophen-2- yl)-3-oxopropanoate derivatives 4f-4i,and di((4-acetyl-3-methyl- 5-phenylamino)thiophen-2-yl)ketone derivatives 4j-4n from readily available acetyl acetone 1,phenyl isothiocynates 2a-2e, and 2-chloromethyl derivatives 3a-3c under mild conditions. The reaction is applicable to a wide range of starting materials. All the synthesized compounds were evaluated for their antibacterial activities against S. aureus,B. subtilis,E. coli,P. aeruginosa,C. albicans,and A. niger microorganisms by the serial dilution method. The synthesized series showed excellent to good activity against Gram-negative micro-organisms (P. aeruginosa and E. coli) and the least activity against Gram-positive bacteria (S. aureus and B. subtilis). All compounds of the series exhibited moderate to less antifungal activity against A. niger and C. albicans.
AcknowledgmentsThe authors are thankful to the Dr. S.B. Jadhav Head,Dept of Pharmaceutical Chemistry,Modern College of Pharmacy Nigdi Pune-44 & Mr. Dnyaneshwar D. Daware for providing necessary help during this work.
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.03.044.
| [1] | A. Dömling, W. Wang, K. Wang, Chemistry and biology of multicomponent reactions, Chem. Rev. 112 (2012) 3083-3135. |
| [2] | A. Dömling, Recent developments in isocyanide based multicomponent reactions in applied chemistry, Chem. Rev. 106 (2006) 17-89. |
| [3] | F.M. Moghaddam, H.Z. Boinee, An efficient and facile one-step synthesis of highly substituted thiophenes, Tetrahedron 60 (2004) 6085-6089. |
| [4] | K.I. Molvi, M. Mansuri, V. Sudarsanam, et al., Synthesis, anti-inflammatory, analgesic and antioxidant activities of some tetrasubstituted thiophenes, J. Enzyme Inhib. Med. Chem. 23 (2008) 829-838. |
| [5] | M.K. Parai, G. Panda, V. Chaturvedi, Y.K. Manju, S. Sinha, Thiophene containing triarylmethanesasantitubercularagents, Bioorg.Med.Chem.Lett.18(2008)289-292. |
| [6] | W. Wardakhan, O. Abdel-Salam, G. Elmegeed, Screening for antidepressant, sedative and analgesic activities of novel fused thiophene derivatives, Acta Pharm. 58 (2008) 1-14. |
| [7] | S. Sharma, F. Athar, M.R. Maurya, A. Azam, Copper(II) complexes with substituted thiosemicarbazones of thiophene-2-carboxaldehyde: synthesis, characterization and antiamoebic activity against E. histolytica, Eur. J. Med. Chem. 40 (2005) 1414-1419. |
| [8] | O.F. William, Principles of Medicinal Chemistry, 3rd ed., Lippincott Williams & Wilkins Publication, Philadelphia, 1989. |
| [9] | A.A. Sagardoy, M.J. Gil, R. Villar, et al., Benzo[b]thiophene-6-carboxamide 1,1-dioxides: inhibitors of human cancer cell growth at nanomolar concentrations, Bioorg. Med. Chem. 18 (2010) 5701-5707. |
| [10] | A.A. Fadda, E. Abdel-Latif, R.E. El-Mekawy, Synthesis and molluscicidal activity of some new thiophene, thiadiazole and pyrazole derivatives, Eur. J. Med. Chem. 44 (2009) 1250-1256. |
| [11] | Indian Pharmacopoeia, Microbiological Assay of Antibiotics, vol. I, 2007, pp. 45-52. |
| [12] | C.R. Kokare, Pharmaceutical Microbiology and Biotechnology, 3rd ed., Nirali Prakashan, Pune, 2006, pp. 21.1-21.12. |
| [13] | Ananthanarayan, Paniker, Textbook of Microbiology, 7th ed., Orient Longman, Chennai, 2005, pp. 628-630. |
| [14] | Y.J. Huang, A. Dömling, The Gewald multicomponent reaction, Mol. Divers. 15 (2011) 3-33. |