b School of Automation, Xi'an University of Posts and Telecommunications, Xi'an 710121, China
1. Introduction
Besides zinc and iron,copper ranks as third among the essential heavy metals in abundance in human bodies [1]. Copper plays an important role in the areas of biological,environmental,and chemical systems [2]. It is an essential trace element for both plants and animals,including humans,but alterations in its cellular homeostasis are connected to serious neurodegenerative diseases, including Menkes and Wilson diseases [3, 4, 5],familial amyotropic lateral sclerosis [6, 7],Alzheimer’s disease [8],and prion diseases [9]. Hence,it is very essential to detect copper.
Fluorescence sensing has wide use in the environment, chemistry,biology,and medicine due to its high sensitivity,high selectivity,fast analysis with spatial resolution,and its sample/cell non-destructive nature [10, 11, 12]. Thus,the design and synthesis of fluorescent copper probes have attracted intense attention,most of which,however,were synthesized by very difficult methods and expensive raw materials [13, 14, 15]. Since coumarin derivatives exhibited several advantages,such as ease of synthesis,not very expensive,large Stokes shifts,good photostability and high fluorescence quantum yields,they could be used as efficient fluorophores [16]. Consequently,in recent years,the design and synthesis of many coumarin-based fluorescent probes for the detection of anions,cations and neutral molecules have been reported [17, 18]. Bai reported the S2- fluorescent sensors based on an 8-hydroxyquinoline-appended fluorescein derivative,which had high selectivity to Cu2+ and exhibited a visible color change [19]. Inspired by the idea,we synthesized a novel fluorescent probe 3 (CQP,Scheme 1) by ethyl-7-hydroxy-8-formylcoumarin-3- carboxylate and 2-(quinolin-8-yloxy)acetohydrazide.

|
Download:
|
| Scheme 1. The synthesis of compound 1 (CQP). | |
The fluorescent metal ion probes which were synthesized by the PET (photo-induced electron transfer) recognition mechanism always bear the selection of suitable energy donors,energy acceptor and linkers [20, 21, 22, 23]. Now,we report one novel fluorescent probe,CQP,which was rationally designed on the basis of a coumarin-quinoline scaffold as the PET mechanism platform. It exhibited strong fluorescence responses and high selectivity toward Cu2+ over other metal ions. With the addition of Cu2+, compound CQP could give rise to a visible colorless-to-yellow color change and clear fluorescence quenching. The binding constant between the compound CQP and Cu2+ was calculated using Benesi- Hildebrand equation [24].
2. ExperimentalThe NMR spectra were measured by Varian Unity INOVA-400 spectrometer with tetramethylsilane (TMS) as internal standard. Infrared (IR) spectra in cm-1 were recorded on a Bruker EQUIOX-55 spectrometer (Germany). Mass spectra were acquired on a Bruker micrOTOF-Q II Mass Spectrophotometer (Germany). All fluorescence measurements were made on a Hitachi F-4500 Fluorescence Spectra (Tokyo,Japan) with excitation slit set at 5.0 nm and emission at 5.0 nm in 1 cm × 1 cm quartz cell. Absorption spectra were measured on Lambda 35 UV/vis spectrophotometer.
All reagents of analytical grade were obtained froma commercial source and used without further purification. Deionized water was used throughout all experiments. The Na2HPO4-citric acid buffer solutions were prepared by using proper amount of Na2HPO4 and citric acid under adjustment by a pH meter. All inorganic salts used were of analytical grade. Co(NO3)2·6H2O,Mn(NO3)2,Al2 (SO4)3, Zn(NO3)2·6H2O,Ca(NO3)2·4H2O,HgCl2,Cu(NO3 )2·3H2O,SnCl2, Ni(NO3)2·6H2O,Cd(NO3)2·2H2O,Cr(NO3)3·9H2O,AgNO3,Fe(NO3)3· 9H2O,Mg(NO3)2·6H2O and Pb(NO3)2 were dissolved in deionized water,but Al2(SO4)3 and Fe(NO3)3·9H2O were dissolved in acidic deionized water.
Synthesis of ethyl-7-hydroxy-8-formylcoumarin-3-carboxylate (compound 2): Ethyl-7-hydroxycoumarin-3-carboxylate (1.64 g, 7 mmol) and hexamine (1.47 g,10.5 mmol) were added to a stirred TFA (15 mL). This solution was refluxed for 24 h. Then,30 mL water was added,and the mixture was warmed to 60 ℃ while stirring for another 30 min. Upon cooling on ice,a yellow solid was precipitated from the solution,collected by filtration and washed several times with water to give the desired compound as a yellow solid (1.25 g,68% yield). IR (KBr,cm-1): 3431,3045,1742,1700, 1659,1595,1480,1445,1304,1236; MS (MALDI-TOF): m/z,calcd. for (M+) 262.22; found 262.20; 1 NMR (400 MHz,DMSO-d6): δ 1.28-1.31 (t,3H,J = 6 Hz),4.25-4.30 (q,2H,J = 6.8 Hz),7.01 (s,1H), 8.06 (m,1H),8.75 (s,1H),10.40 (s,1H),12.50 (s,1H).
Synthesis of (E)-ethyl-7-hydroxy-8-[[2-[2-(quinolin-8-yloxy)acetyl] hydrazono]methyl]-coumarin-3-carboxylate (1,CQP): The 2- (quinolin-8-yloxy)acetohydrazide 3 was prepared by the reported method [23]. Synthetic routes of CQP are depicted in Scheme 1 and it was fully characterized by 1 NMR,13C NMR,IR and mass spectrum. Briefly,compound 3 was dissolved in 25 mL of ethanol, followed by the addition of compound 2. Then the mixture solution was stirred at 65 ℃ for 6 h. The cream-colored precipitate formed was filtered and washed with ethanol and acetone and dried under vacuum,then compound CQP was obtained (Scheme 1). Yield: 78%. 1 NMR (400 MHz,DMSO-d6): δ 1.31 (t,3H,J = 4 Hz),4.29 (m,2H), 4.89 (s,1H),5.76 (s,2H),7.01 (dd,1H,J = 6 Hz),7.36 (dd,1H, J = 6 Hz),7.48 (t,1H,J = 8 Hz),7.86 (m,4H),8.77 (s,1H),9.12 (s,1H). 13C NMR (100 MHz,DMSO-d6): δ 169.8,168.5,167.7,160.5,159.8, 158.7,154.7,154.3,148.4,141.3,138.3,134.2,131.9,127.2,126.4, 119.5,117.0,115.4,110.2,73.4,66.1,19.25. FI-IR (KBr,cm-1): 3521,3419,3056,2971,1732,1688,1615,1583,1506,1476,1374, 1312,1236,1113,1089,1025,952,816,755,685,643,596,523, 487. HRMS (ESI): m/z,calcd. for C24H29N3O7; 462.1301 [M+H]+; found 462.1290.
Stock solutions of various ions (1.0 × 10-3 mol/L) were prepared in deionized water. A stock solutions of CQP (1 × 10-3 mol/L) were prepared in DMSO/methanol (1:5,v/v). The solution of CQP was then diluted to 1 × 10-4 mol/L with methanol water. A standard test solution was prepared by dilution of the solution of CQP (1 × -4 mol/L). A stock solution of cupric sulfate (1 × 10-2 mol/L) was prepared by dissolving 14.37 mg of cupric sulfate in 50.0 mL water. A standard test solution was prepared by dilution of the solution of cupric sulfate (1 × 10-2 mol/L). Na2HPO4-citric acid buffer solutions (0.2 mol/L, pH 7.4) were employed in all test.
3. Results and discussionFree CQP shows absorption at 275 nm and 385 nm in ethanol. Upon the gradual addition of Cu2+,the intensity of the absorption at 275 nm and 385 nm increased while at the same time the hypochromatic shift appeared 50 nm and 20 nm,respectively,in Fig. 1,resulting in a color change from colorless to yellow. Other metal cations did not show the same phenomenon.

|
Download:
|
| Fig. 1. The absorption spectra of CQP and CQP + Cu2+. The red line represents only the CQP (10 μmol/L),the black line represents the subsequent addition of Cu2+(10 mmol/L) to the solution. The buffer solution was Na2HPO4-citric acid buffer(10 mmol/L,pH 7.4). | |
The effect of pH on the fluorescent response of CQP to Cu2+ was studied in a concentration of Na2HPO4-citric acid fixed at 10 mmol/L,and the result shown in Fig. 2. It can be seen that the fluorescence emission of the free CQP was stable from pH 2.2 to 7.0,however,the fluorescence emission at pH > 7 slightly increase, at the same time the slight hypochromatic shift can be seen in Fig. 2a,which can be ascribe to the state of proton of the hydroxyl in CQP. Upon the addition of Cu2+,the fluorescence emission of CQP-Cu2+ increased quickly in an acidic environment (from pH 2.2 to 6.4) and increased rapidly in an alkaline environment (pH > 7), however,the fluorescence emission slightly decreased when pH > 8 in Fig. 2b,possible because the hydroxyl could tautomerize to quinone to delay the electron transform. The slight hypochromatic shift can be seen in Fig. 2b,which may due to the state of proton of the hydroxyl in CQP. The fluorescent intensity changes with the different pH were shown in Fig. 2c. In order to obtain a higher signal-to-noise ratio,Na2HPO4-citric acid buffer (pH 7.4, 10 mmol/L) was employed for the Cu2+ assay throughout the experiment.

|
Download:
|
| Fig. 2. (a) The effect of pH on the fluorescent intensity of CQP (10 μmol/L); (b) the effect of pH on the fluorescence intensity of CQP (10 μmol/L) upon the addition of Cu2+ (10 μmol/L); (c) the effect of pH on the fluorescent intensity of CQP and CQP + Cu2+. The red line represents CQP only,the black line represents the subsequent addition of Cu2+ to the solution. The buffer solution was Na2HPO4-citric acid buffer (10 mmol/L,pH 7.4). | |
To obtain insight into the fluorescent properties of CQP toward metal ions,the emission changes were investigated with different ions,such as Al3+,Mg2+,Sn2+,Ca2+,Fe3+,Hg2+,Mn2+,Cd2+,Cr3+,Zn2+, Co2+,Ni2+,Ag+,Pb2+and Cu2+ in Na2HPO4-citric buffer (10 mmol/L, pH 7.4). The emission spectra were investigated by exciting CQP at 410 nm in Fig. 3a. From the emission spectra,it can be seen that there was a great decrease in the fluorescence intensity of Cu2+ with CQP,which could be clearly distinguished from the other metal ions in Fig. 3a. With the addition of Cu2+,compound CQP could give rise to a visible colorless-to-yellow color change in Fig. 4a and the prominent fluorescence changes of CQP were also observable by a hand-gun UV lamp in Fig. 4b. The weak yellowgreen color that came from the solution of CQP faded away only by the addition of Cu2+. The light blue fluorescence was only observed with CQP-Cu2+. The combined information of fluorescence and visible changes will be useful to discriminate Cu2+ from other metal ions.

|
Download:
|
| Fig. 3. (a) Fluorescence emission spectra of CQP (10 mmol/L) in the presence of different ions such as Al3+,Mg2+,Sn2+,Ca2+,Fe3+,Hg2+,Mn2+,Cd2+,Cr3+,Zn2+,Co2+,Ni2+,Ag+,Pb2+ and Cu2+ in Na2HPO4-citric acid buffer (10 mmol/L,pH 7.4). (b) Metal ions selectivity of CQP (10 mmol/L) toward Cu2+ in Na2HPO4-citric acid buffer (10 μmol/L,pH 7.4). The concentration of Cu2+ was 10 mmol/L,and that of the other metal ions 15 μmol/L. Red bars represent the addition of the appropriate metal ions to the solution of CQP. Black bars represent the subsequent addition of Cu2+ to the solution. | |

|
Download:
|
| Fig. 4. (a) The color changes of CQP and CQP + Cu2+; (b) the color changes of CQP and CQP + Cu2+ under the irradiation at 365 nm. | |
In order to validate the high sensitivity of CQP to Cu2+ in practice,competition experiments were carried out by adding Cu2+ to the solution of CQP in the presence of Al3+,Mg2+,Sn2+,Ca2+,Fe3+, Hg2+,Mn2+,Cd2+,Cr3+,Zn2+,Co2+,Ni2+,Ag+,Pb2+ and Cu2+ in Fig. 3b. It was observed that the presence of metal ions such as Al3+,Mg2+, Sn2+,Ca2+,Fe3+,Hg2+,Mn2+,Cd2+,Cr3+,Zn2+,Co2+,Ni2+,Ag+,Pb2+ did not interfere with the CQP-Cu2+ decreased fluorescence. However, the presence of Ca2+,Cr3+ and Zn2+ clearly increase the fluorescence intensity,possible because the complex formed between these metals and the probe was too stable to be replaced by Cu2+, indicating that more attention should be paid to study the probe.
Further,the binding affinity of CQP for Cu2+ was examined through a fluorescence titration experiment in which different concentrations of Cu2+ were added to the solution of CQP (10 mmol/L) under the condition (Na2HPO4-citric acid,10mmol/L, pH 7.4,λex = 410 nm). Upon the addition of Cu2+ to the solution of CQP,fluorescence decrease occurred and the emission intensity at 443 nm clearly decreased in Fig. 5a. Moreover,the linear relationships of CQP toward Cu2+ at the low concentration range (see insertion in Fig. 5a),showed that a reasonable linear relationship could be built when the concentration of Cu2+ did not exceed that of the CQP. To study the binding of CQP with Cu2+,the Job’s plot was tested by using a total concentration of 10 mmol/L the CQP and Cu2+, and the results indicated that the probes combinedwith Cu2+ with a 1:1 stoichiometry in Fig. 5b.

|
Download:
|
| Fig. 5. (a) Fluorescent changes of CQP (10 μmol/L) with different concentrations of Cu2+ (0,1,2,3,4,5,6,7,8,9,10,11,12,13,14,15 μmol/L,respectively) in buffer solution (Na2HPO4-citric acid,pH 7.4) with an excitation at 410 nm. Insert figure: the linear relationships of CQP toward Cu2+ (below 10 mmol/L). (b) Job’s plot of CQP-Cu2+. The total concentration of CQP and Cu2+ was kept constant at 10 μmol/L in Na2HPO4-citric acid buffer (10 mmol/L,pH 7.4). The excitation was at 410 nm (μmol/L). | |
The binding ability of CQP toward Cu2+ was calculated following the modified Benesi-Hildebrand equation (fluorescence method)

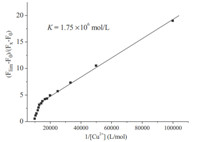
where ΔF = (Fx - F0),ΔFmax = (Flim - F0) and F0,Fx,Flim are the emission intensities of CQP with adding Cu2+ at an intermediate Cu2+ concentration,and at the concentration of complete interaction, respectively. K is the binding constant between compound CQP and Cu2+. C is the Cu2+ concentration and n is the number of Cu2+ bound per CQP (here,n = 1). The value of K can be obtained from the slope in Fig. 6. The results obtained were KCQP = 1.75 × 106 mol/L,signifying that CQP may has a great banding affinity for Cu2+.
|
Download:
|
| Fig. 6. Determination of binding constant of CQP (10 μmol/L) with Cu2+ (10 μmol/L)using Benesi-Hildebrand equation (fluorescent method). | |
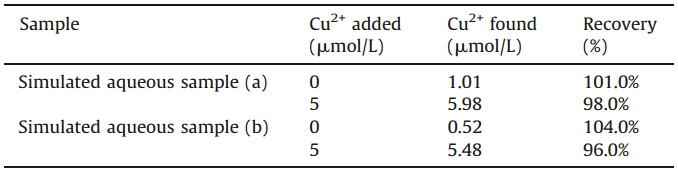
Simulated aqueous sample (a) obtained by deionized water, 1.0 μmol/L CuSO4,5.0 μmol/L CdCl2,20 μmol/L NaCl,KCl,MgSO4, and CaCl2 and simulated aqueous sample (b) obtained by deionized water,1.0 μmol/L CuSO4,5.0 μmol/L Ni(NO3)2,20μmol/L NaCl, KCl,MgSO4,were analyzed by the proposed probe. Different amount of simulated aqueous sample were added to the solution of compound 1 in Na2HPO4-citric acid buffer (10 mmol/L,pH 7.4). As shown in Fig. S1 in Supporting information,a linear relationship between fluorescent intensity and the volume of simulated aqueous sample was noted,thus indicated that the compound 1 is capable of detection Cu2+ in simulated aqueous sample. Simulated aqueous sample were analyzed by compound 1 under optimized conditions (Table 1). From the above results,it can be seen that compound 1 can measure the concentration of Cu2+ in water samples with good recovery results. Therefore,compound 1, CQP,can be employed for Cu2+ assay in a water setting.
| Table 1 Determination of Cu2+ concentrations in water samples. |
In summary,on the basis of PET mechanism,we present a Schiff-base fluorescent probe for the detection of Cu2+ based on coumarin. The probe displayed an apparent response to Cu2+ via a significant fluorescent change and a color change from colorless to yellow. Moreover,other metal ions have no effect on its specific response to Cu2+. The 1:1 binding mode was proposed based on the Job’s plot. Also,the binding constant between CQP and Cu2+ was calculated by Benesi-Hildebrand equation (fluorescent method). The probe can be employed to measure Cu2+ in a water setting.
AcknowledgmentsThis work was supported by the National Natural Scientific Foundation of China (Nos. 21072158 and 20802056),the Natural Science Foundation of Shaanxi Province (No. 2012JM8022),the Special Foundation of the Education Committee of Shaanxi Province (No. 12JK0580),the French Chinese Foundation for Science and Applications (FFSCA) and the China Scholars Council (CSC).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet. 2014.05.001.
| [1] | Y. Lin, W.Y. Lin, B. Chen, Y.A. Xie, Development of FRET-based ratiometric fluorescent Cu2+ chemodosimeters and the applications for living cell imaging, Org. Lett. 14 (2012) 432-435. |
| [2] | J.L. Fan, P. Zhan, M.M. Hu, et al., A fluorescent ratiometric chemodosimeter for Cu2+ based on TBET and its application in living cells, Org. Lett. 12 (2013) 492-495. |
| [3] | D.J. Waggoner, T.B. Bart, J.D. Gitlin, The role of copper in neurodegenerative disease, Neurobiol. Dis. 6 (1999) 221-230. |
| [4] | C. Vulpe, B. Levinson, S. Whitney, S. Packman, J. Gitschier, Isolation of a candidate gene for Menkes disease and evidence that it encodes a copper-transporting ATPase, Nat. Genet. 3 (1993) 7-13. |
| [5] | P.C. Bull, G.R. Thomas, J.M. Rommens, J.R. Forbes, D.W. Cox, The Wilson disease gene is a putative copper transporting P-type ATPase similar to the Menkes gene, Nat. Genet. 5 (1993) 327-337. |
| [6] | J.S. Valentine, B.G. Hart, J.R. Winkler, Copper(II) binding to alpha-synuclein, the Parkinson's protein, Proc. Natl. Acad. Sci. U.S.A. 130 (2008) 6898-6899. |
| [7] | N. Shao, J.Y. Jin, H. Wang, et al., Tunable photochromism of spirobenzopyran via selective metal ion coordination: an efficient visual and ratioing fluorescent probe for divalent copper ion, Anal. Chem. 80 (2008) 3466-3475. |
| [8] | G. Meloni, P. Faller, M. Vasak, Redox silencing of copper in metal-linked neurodegenerative disorders: reaction of Zn7metallothionein-3 with Cu2+, J. Biol. Chem. 282 (2007) 16068-16078. |
| [9] | T.Y. Han, Y.F. Dong, Z.Y. Li, et al., Turn-on fluorescent detection of 2,5-di(4'-carboxylphenyl)-1-phenylpyrrole to amines, Acta Chem. Sin. 70 (2012) 1187-1192. |
| [10] | X.B. Li, Z.G. Niu, L.L. Chang, M.X. Chen, E.J. Wang, Quinoline-based colorimetric chemosensor for Cu2+: Cu2+ induced deprotonation leading to color change, Chin. Chem. Lett. 25 (2014) 80-82. |
| [11] | Z.C. Xu, X.H. Qian, J.N. Cui, Colorimetric and ratiometric fluorescent chemosensor with a large red-shift in emission: Cu(II)-only sensing by deprotonation of secondary amines as receptor conjugated to naphthalimide fluorophore, Org. Lett. 7 (2005) 3029-3032. |
| [12] | Ulf-P. Apfel, D. Buccella, J.J. Wilson, S.J. Lippard, Detection of nitric oxide and nitroxyl with benzoresorufin-based fluorescent sensors, Inorg. Chem. 52 (2013) 3285-3294. |
| [13] | Y. Fu, C.Q. Ding, A.W. Zhu, et al., Two-photon ratiometric fluorescent sensor based on specific biomolecular recognition for selective and sensitive detection of copper ions in live cells, Anal. Chem. 85 (2013) 11936-11943. |
| [14] | Y. Zhao, X.B. Zhang, Z. Han, et al., Highly sensitive and selective colorimetric and off-on fluorescent chemosensor for Cu2+ in aqueous solution and living cells, Anal. Chem. 81 (2009) 7022-7030. |
| [15] | D.M. Pluth, L.E. McQuade, S.J. Lippard, Cell-trappable fluorescent probes for nitric oxide visualization in living cells, Org. Lett. 12 (2010) 2318-2321. |
| [16] | J.Y. Jo, H.Y. Lee, W.J. Liu, et al., Reactivity-based detection of copper (II) ion in water: oxidative cyclization of azoaromatics as fluorescence turn-on signaling mechanism, J. Am. Chem. Soc. 134 (2012) 16000-16007. |
| [17] | S.J. Hyo, H.H. Ji, H.K. Zee, H.K. Chul, S.K. Jong, Coumarin-Cu (II) ensemble-based cyanide sensing chemodosimeter, Org. Lett. 13 (2011) 5056-5059. |
| [18] | C.J. Kirubaharan, D. Kalpana, Y.S. Lee, et al., Biomediated silver nanoparticles for the highly selective copper(II) ion sensor applications, Ind. Eng. Chem. Res. 51 (2012) 7441-7446. |
| [19] | F.P. Hou, L. Huang, P.X. Xi, A retrievable and highly selective fluorescent probe for monitoring sulfide and imaging in living cells, Inorg. Chem. 51 (2012) 2454-2460. |
| [20] | A. Coskun, E.U. Akkaya, Ion sensing coupled to resonance energy transfer: a highly selective and sensitive ratiometric fluorescent chemosensor for Ag (I) by a modular approach, J. Am. Chem. Soc. 127 (2005) 10464-10465. |
| [21] | J.B. Wang, X.H. Qian, A series of polyamide receptor based PET fluorescent sensor molecules: positively cooperative Hg2+ ion binding with high sensitivity, Org. Lett. 8 (2006) 3721-3724. |
| [22] | A.P. de Silva, A.H.Q. Gun, L.T. Gunn, et al., Signaling recognition events with fluorescent sensors and switches, Chem. Rev. 97 (1997) 1515-1566. |
| [23] | X. Zhao, M.X. Jia, X.K. Jiang, et al., Zipper-featured d-peptide foldamers driven by donor-acceptor interaction. Design, synthesis, and characterization, J. Org. Chem. 69 (2004) 270-279. |
| [24] | H.A. Benesi, J.H. Hildebrand, A spectrophotometric investigation of the interaction of iodine with aromatic hydrocarbons, J. Am. Chem. Soc. 71 (1947) 2703-2707. |