b Key Laboratory of Medicinal Chemistry and Molecular Diagnosis of Ministry of Education, Hebei University, Baoding 071002, China;
c Department of Immunology, School of Basic Medical Science, Hebei University, Baoding 071002, China
The human telomeric G-quadruplexes DNA refers to the four stranded structures formed by the guanine-rich DNA sequences, which presents in the telomeres of eukaryotic chromosomes [1]. Telomeres have a crucial function in restricting the proliferative capacity of normal cells. However in most tumor cells,telomerase activity has been acquired and over-expressed,which resulted in the unlimited growth of the tumor cells [2]. In recent years,Gquadruplexes DNA has become a potential target for new anticancer drugs [3, 4, 5]. The G-quadruplex ligands can induce DNA molecules to form stable G-quadruplex structures,then to inhibit the activity of the telomerase,resulting in rapid apoptosis of the cancer cell [3, 4]. In order to improve the selectivity and reduce the side effects of DNA-interactive anticancer drugs,many researchers have focused their interests on designing and synthesis of DNA G-quadruplex ligands [6, 7, 8, 9]. To date,several drugs based on DNA G-quadruplex ligands have been in clinical trials,such as Quarfloxin and BRACO-19 (Fig. 1) [10, 11].

|
Download:
|
| Fig. 1.The structures of some G-quadruplex ligands and antitumor drugs based on the anthraquinone derivatives. | |
Anthraquinone derivatives are one of the earliest G-quadruplex DNA ligands. For example,BSU1051 (Fig. 1) can inhibit the telomerase activity through π-π interactions with the terminal G-tetrad of a quadruplex,and the electrostatic interaction between the nitrogen atom and phosphate ester of the side chains and the G-quadruplex grooves. In order to improve the binding capacity and selectivity of ligand to G-quadruplex DNA by synergy of multi-site and multimode,scientists paid more attention to the synthesis of anthraquinone derivatives. Stephen Neidle et al. reported on a series of DNA G-quadruplex ligands based on anthraquinone derivatives,and found that 2,6- and 2,7-bis aminoacyl substituted anthraquinones showed a preference for triplex and quadruplex DNA vs. duplex DNA [12].
It is well known that carbohydrates play diverse and crucial roles in a wide variety of biological systems [13]. The anthraquinone and carbohydrate conjugates,as anticancer drugs,have been widely and successfully used in the clinic,such as Doxorubicin and Sabarubicin (Fig. 1),and an investigation has also revealed that the glycosyl moiety could improve the selectivity of the G-quadruplex DNA [14]. On the other hand,azasugar (iminosugar),as a kind of carbohydrate mimic,has remarkable biological properties [15], especially because its nitrogen atom can interact with the phosphate ester chain by electrostatic interaction under physiological conditions. Ranjan and coworkers found that aminosugars were also identified as G-quadruplex DNA ligand by binding with the quadruplex grooves [16]. However,the glycosyl modified G-quadruplex ligands have rarely been reported. Herein we designed and synthesized series novel azasugar-modified anthraquinones derivatives at 2-,2,6- and 2,7-positions with different lengths of the linking chains. Their structures were characterized by NMR and HRMS analysis. Their cytotoxic activities against human epithelial cervical cell (HeLa) and human breast cancer cells (MCF-7) were preliminarily evaluated. 2. Experimental 2.1. Instruments
Melting points were measured on a SGW X-4 micro melting point apparatus and are uncorrected. 1H NMR,13C NMR spectra were measured on a RT-NMR Bruker AVANCE 600 (600 MHz) spectrometer using tetramethylsilane (Me4Si) as the internal standard. High-resolution mass spectra (HRMS) were recorded on a FTICR-MS (Ionspec 7.0T) mass spectrometer in the electrosprayionization (ESI) mode. The microwave assisted reactions were carried out on a DISCOVER S-Class Auto Focused Microwave Synthesis System (CEM Corporation,USA). Thin-layer chromatography (TLC) was performed on precoated plates (Qingdao GF254) with detection by UV light or with phosphomolybdic acid in EtOH/ H2O followed by heating. Column chromatography was performed using a SiO2 (Qingdao 300-400 mesh). 2.2. Synthesis and cytotoxicity analyses
Compounds 5a-c N-alkylamino azasugars were synthesized using D-mannitol as the starting material,benzylidenation and bistrifluoromethanesulphonation of D-mannitol to obtain compound 3. Then nucleophilic substitution of N-Boc-diamine to compound 3 produced the N-Boc-alkylamino benzylidene azasugars 4a-c. Following the deprotection the N-alkylamino azasugars 5a-c were obtained. Then through the nucleophilic substitution of N-alkylamino azasugar 5a-c with the corresponding chloroacetamidoanthraquinone (6,7,8),the targeted compounds 9-11 were obtained (experimental procedures and characterization data for compounds 4-11 and cytotoxicity analyses,please see Supporting information). 3. Results and discussion
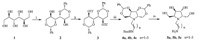
N-Alkylamino azasugars 5a-c were synthesized using Dmannitol as the starting material (Scheme 1). Compound 3 was obtained through benzylidenation and bistrifluoromethanesulphonation of D-mannitol according to the procedure in the literature [17]. Then nucleophilic substitution of N-Boc-diamine to compound 3 produced the N-Boc-alkylamino benzylidene azasugars 4a-c in yields of 78%-80%. Then compounds 4a-c in a solution of concentrated hydrochloric acid/1,4-dioxane (1:1,v/v) were refluxed for 24 h to remove the benzylidene and Boc group and afford the N-alkylamino azasugars 5a-c in yields of 75%,77% and 76%,respectively.
|
Download:
|
| Scheme 1.Synthesis of N-alkylamino azasugars 5a-c. Reagents and conditions: (i) benzaldehyde,H2SO4,DMF,r.t. 5 d; (ii) Tf2O,Py.,CH2Cl2; (iii) N-Bocdiamine,THF,TEA, reflux,78%-82%; (iv) hydrochloric acid/1,4-dioxane (1:1,v/v),reflux,75%-77%. | |
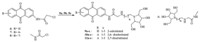
The starting materials,2-aminoanthraquinone and 2,6-diaminoanthraquinone, are commercially available,and the 2,7-diaminoanthraquinone was prepared from anthrone according to the literature [18]. Compounds 6-8 were prepared by the acylation from the corresponding aminoanthraquinones with chloroacyl chloride according to the reported procedure [18],followed by the nucleophilic substitution with the N-alkylamino azasugar, 5a-c,to afford the targeted compounds 9-11 (Scheme 2). The nucleophilic substitution of 2-chloroacetamidoanthraquinone (6) and N-ethylamino azasugar 5a was studied to optimize the reaction conditions with different solvents,temperature,the acidbinding agent,the amount of additive KI,as well as microwave irradiation. It was found that although the reaction with normal heating at 80 ℃ for 24 h provided compound 6 only in 10% yield, the microwave irradiation could remarkably improve the reaction efficiency with a few minutes of irradiation. Thus,the microwave assisted reaction of compound 6 and N-alkylamino azasugar 5a (2.0 equiv.) was carried out in DMF,in the presence of DIPEA and KI,under microwave irradiation at 55 ℃ for 20 min to afford compound 9 in a yield of 60%. Similarly,compounds 9b-11c were obtained in yields of 30%-59%.
|
Download:
|
| Scheme 2.Synthesis of compounds 9-11. Reagents and conditions: DMF,DIPEA,KI,M.W.,55 ℃,20 min,30%-60%. | |
The cytotoxicity of the novel,azasugar-modified anthraquinone derivatives 9-11 against HeLa cell lines (human cervical cancer cells) and MCF-7 (human breast cancer cells) in vitro were examined with comparison to the control drug (cisplatin). As shown in Table 1,the compounds showed lower cytotoxic activity against HeLa and Mcf-7 cells,however,compounds 9a and 10b exhibit good selective toxicity against MCF-7,and especially compound 9a with mono-azasugar conjugate at the 2-position exhibited a similar activity to MCF-7 as the control drug (cisplatin). For all compounds,the mono- and di-substituted position and the lengths of the chain between anthraquinone and azasugar all influenced the cytotoxic activities.
| Table 1 The cytotoxic activities of compounds 9-11. |
A series of novel,azasugar-modified anthraquinone derivatives at 2-,2,6- and 2,7-positions have been synthesized. Their cytotoxic activities against HeLa and MCF-7 cells were preliminarily evaluated. Compound 9a with the mono-azasugar conjugate at the 2-position showed good activity against MCF-7. The further synthesis,biological activities and DNA binding properties of carbohydrate modified anthraquinone derivatives are underway.
AcknowledgmentsThis work was supported by the National Natural Science Foundation of China (Nos. 21372059 and 21172051),the Hebei Key Basic Research (No. 12966417D),the Hebei Natural Science Foundation (No. B2012201041) and the Foundation of Hebei Education Department (No. YQ2013006).
Appendix A. Supplementary dataSupplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.05.029.
| [1] | M.L. Bochman, K. Paeschke, V.A. Zakian, DNA secondary structures: stability and function of G-quadruplex structures, Nat. Rev. Genet. 13 (2012) 770-780. |
| [2] | N.W. Kim, M.A. Piatyszek, K.R. Prowse, et al., Specific association of human telomerase activity with immortal cells and cancer, Science 266 (1994) 2011-2015. |
| [3] | S. Müller, S. Kumari, R. Rodriguez, S. Blasubramanian, Small-molecule-mediated G-quadruplex isolation from human cells, Nat. Chem. 2 (2010) 1095-1098. |
| [4] | D. Koirala, S. Dhakal, B. Ashbridge, et al., A single-molecule platform for investigation of interactions between G-quadruplexes and small-molecule ligands, Nat. Chem. 3 (2011) 782-787. |
| [5] | S. Lin, M. Xu, G. Yuan, Study of STAT3 G-quadruplex folding patterns by CD spectroscopy and molecular modeling, Chin. Chem. Lett. 23 (2012) 329-331. |
| [6] | D. Sun, B. Thompson, B.E. Cathers, et al., Inhibition of human telomerase by a G-quadruplex-interactive compound, J. Med. Chem. 40 (1997) 2113-2116. |
| [7] | M.P. Teulade-Fichou, C. Carrasco, L. Guittat, et al., Selective recognition of G-quadruplex telomeric DNA by a bis(quinacridine) macrocycle, J. Am. Chem. Soc. 125 (2003) 4732-4740. |
| [8] | T. Lemarteleur, D. Gomez, R. Paterski, et al., Stabilization of the c-myc gene promoter quadruplex by specific ligands' inhibitors of telomerase, Biochem. Biophys. Res. Commun. 323 (2004) 802-808. |
| [9] | J.H. Tan, L.Q. Gu, J.Y. Wu, Design of selective G-quadruplex ligands as potential anticancer agents, Mini Rev. Med. Chem. 8 (2008) 1163-1178. |
| [10] | S.M. Hampel, A. Sidibe, M. Gunaratnam, J.F. Riou, S. Neidle, Tetrasubstituted naphthalene diimide ligands with selectivity for telomeric G-quadruplexes and cancer cells, Bioorg. Med. Chem. Lett. 20 (2010) 6459-6463. |
| [11] | M. Micco, G.W. Collie, A.G. Dale, et al., Structure-based design and evaluation of naphthalene diimide G-quadruplex ligands as telomere targeting agents in pancreatic cancer cells, J. Med. Chem. 56 (2013) 2959-2974. |
| [12] | G. Zagotto, C. Sissi, L. Lucatello, et al., Aminoacyl-anthraquinone conjugates as telomerase inhibitors: synthesis, biophysical and biological evaluation, J. Med. Chem. 51 (2008) 5566-5574. |
| [13] | P. Sears, C.H. Wong, Carbohydrate mimetics: a new strategy for tackling the problem of carbohydrate-mediated biological recognition, Angew. Chem. Int. Ed. 38 (1999) 2300-2324. |
| [14] | I. Manet, F. Manoli, B. Zambelli, et al., Affinity of the anthracycline antitumor drugs doxorubicin and sabarubicin for human telomeric G-quadruplex structures, Phys. Chem. Chem. Phys. 13 (2011) 540-551. |
| [15] | N. Ranjan, K.F. Andreasen, S. Kumar, D. Hyde-Volpe, D.P. Arya, Aminoglycoside binding to oxytricha nova telomeric DNA, Biochemistry 49 (2010) 9891-9903. |
| [16] | P. Compain, O.R. Martin, Iminosugars: From Synthesis to Therapeutic Applications, John Wiley and Sons, Ltd, Chichester, 2007, pp. 87-130. |
| [17] | K. Tony, M. Shing, A short and practical synthesis of (2S,5S)-bishydroxymethyl-(3R,4R)-bishydroxypyrrolidine, J. Chem. Soc. Chem. Commun. (1987) 262-263. |
| [18] | S. Venitt, C. Crofton-Sleigh, M. Agbandje, T.C. Jenkins, S. Neidle, Anthracene-9, 10-diones as potential anticancer agents: bacterial mutation studies of amidosubstituted derivatives reveal an unexpected lack of mutagenicity, J. Med. Chem. 41 (1998) 3748-3752. |





