b The College of Life Sciences, State Key Laboratory of Virology, Modern Virology Research Center, Wuhan University, Wuhan 430072, China;
c Wuhan University School of Pharmaceutical Sciences, Wuhan 430071, China
Gossypol,a polyphenolic dialdehyde,is a naturally occurring yellow pigment found in high concentration in the pigment glands of the cotton plant gossypium [1]. Gossypol is marginally toxic,and this toxicity has been associated with the aldehyde groups,thus stimulating interest in exploration of derivatives or analogs of gossypol in which the aldehyde groups are altered [2]. As a result,a large number of gossypol derivatives have been prepared and characterized. Many of these derivatives and analogs possess a diverse array of unusual disease-inhibiting activities as antimalarial, antiparasitic,anticancer,and antiviral activities [3]. Gossypol was reported in 1989 as an anti-HIV agent. However,it was stated that it was unlikely to be a primarily reverse transcriptase (RT) inhibitor,although no data was presented regarding the effects of gossypol in any assay [4]. In a later article,the results suggested that gossypol was,in part,a reverse transcriptase inhibitor. Certainly,the anti-HIV activity of gossypol could not be attributed solely to its RT inhibition [5]. As such,its anti-HIV mechanism still needs further investigation. Two pure enantiomers of gossypol were also evaluated for their capability to inhibit HIV-1. The (-) enantiomer of gossypol (but not the (+) enantiomer of gossypol) had good antiviral activity against HIV-1 in PBM cells [4]. Lin and co-workers had tested a group of compounds related to gossypol for anti-HIV-1 activity and cytotoxicity. Their data supported the conclusion that the aldehyde groups in such compounds were not necessary for anti-HIV-1 activity but contributed strongly to cytotoxicity. Nevertheless,these gossypol derivatives were less active against HIV-1 than some analogs of gossypol [6]. Since then,there have been very few,if any,new reports on the anti-HIV-1 activity of gossypol derivatives.
In our group,some hydrazine-based gossypol Schiff base derivatives and aliphatic gossypol Schiff base derivatives were synthesized and tested for in vitro anti-H5N1 and anti-HIV-1 activities respectively,but the IC50 values of these derivatives were not determined because of higher cytotoxicity on MTT cells or TZM-bl cells. We postulated that the high cytotoxicity of gossypol might limit further studies on its antiviral effects,so we tried to modify the aldehyde groups of gossypol by using hydrophilic groups in order to reduce its cytotoxicity. Recently,our study results indicated that substitution of certain amino acids for the aldehyde groups of gossypol not only reduced the cytotoxicity of gossypol but also enhanced the antiviral activities of gossypol against HIV-1 and H5N. These amino acids include L-alanine, L-valine,2-aminoisobutyric acid,and L-isoleucine [7]. Further study indicated that amino acid derivatives of (-)-gossypol could bind to the gp41 hydrophobic pocket and block the formation of the cell fusion-activated gp41 core to inhibit HIV-1 mediated membrane fusion and subsequent viral entry [8]. At present,T20 (brand name,Fuzeon) is the first and only clinically approved HIV- 1 fusion inhibitor,but the clinical application of T20 has been constrained due to its lack of oral bioavailability and high production costs [9, 10]. Therefore,it is critical to develop novel non-peptide small molecule HIV-1 entry inhibitors.
To further understand the function of substituents of aldehyde groups in gossypol and to find novel derivatives as HIV-1 entry inhibitors,we decided to further modify the aldehyde groups of gossypol by using other hydrophilic groups,such as certain oligopeptides and D-glucosamine,and investigated in vitro anti- HIV-1 activity of the resulting novel compounds. 2. Experimental 2.1. Chemistry
All commercial reagents and solvents used for the reactions were commercially available and were used without further purification. Starting from D-glucosamine,1,3,4,6-tetra-O-acetyl-β- D-glucosamine was prepared according to the reported reference [11] and the spectral data were in full agreement with its structure. Melting points were determined on an XT-4 microscopic melting point apparatus and were uncorrected. IR spectra were recorded from KBr pellets at a range of 400-4000 cm-1 on a Thermo Nicolet Nexus 470 FT-IR spectrometer and were listed in wave numbers (cm-1). 1H NMR and 13C NMR spectra were obtained on a Bruker DPX400 apparatus or Varian Mercury VX-300 apparatus in DMSO-d6 or CDCl3. Chemical shifts were listed in ppm using tetramethylsilane as internal standard. MS spectra were assayed on LC/ MSD Trap SL Plus Mass Spectrometer.
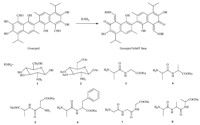
The procedure for synthesis of compounds 1-8 is outlined in Scheme 1. Novel gossypol derivatives were synthesized by treating gossypol with the corresponding oligopeptides and D-glucosamine in a suitable solvent to form adducts in high yields (more than 85%). Both analytical and spectral data of all synthesized compounds were in full agreement with the proposed structures [12].
|
Download:
|
| Scheme 1.Reagents and conditions: (i) for 1-2: methanol,r.t.,3 h,nitrogen; (ii) for 3-8: methanol,NaOH,pH = 7.4,r.t.,3 h,nitrogen. | |
The inhibitory activities of novel compounds against the laboratory-adapted HIV-1ⅢB strain were examined using the HIV-1ⅢB/TZM-bl indicator cell culture system. In short,300 μL of TZM-bl cells at 5 × 103/mL was added into each well of the 48- well plate at 37 ℃ overnight. Then,the cells were infected by the HIV-1ⅢB at a multiplicity of infection (M.O.I.) of 0.1 and incubated at 37 ℃ for 2 h either with the novel compound or not. After each well was washed 3 times with PBS to remove the uninfected virus and novel compound,300 mL fresh medium without the test compound was added into each well. Forty-eight hours later,the cells were lysed by lysis buffer,followed by addition of aliquots of luciferase substrate (Promega). The luciferase activities were quantified using a luciferase reporter assay system (Promega, Madison,I). The percentage of the compound inhibitory activity was calculated by the following formula: [1 - (E - N)/ (P - N)] × 100%. N represents the quantified luciferase activity of the negative control,while P corresponds to that in the positive control. E corresponds to the experimental group. IC50 (the concentration for 50% inhibition) values could be calculated [8]. 2.2.2. Cytotoxicity assays
The in vitro cytotoxicities of the novel compounds on TZM-bl were determined by the MTT colorimetric assay. Concisely,100 μL of a compound at graded concentrations was added to equal volumes of cells (5 × 105/mL) in each well of 96-well plates. After incubation at 37 ℃ for 72 h,100 μL supernatant was discarded and 10 μL MTT solution (5 mg/mL in PBS) was added to each well, followed by incubation at 37 ℃ for 4 h. Then,100 μL of 50% DMF- 20% SDS was added. After the formazan was dissolved completely, the plates were read on a Bio-Tek ELx 800 ELISA reader at 595/ 630 nm. The 50% cytotoxicity concentrations (CC50) were calculated by the software Calcusyn [8]. 2.2.3. Binding to HAS
The binding constants (KAs) of novel gossypol derivatives with human serum albumin (HSA) were determined by fluorescence quenching titrations [13]. 2.2.4. Time-of-addition assays
TZM-bl cells,seeded in 48-well dishes,were incubated with HIV-1ⅢB at 37 ℃ for 0,2,4,and 8 h,before the addition of a test compound (10 μmol/L). AZT and T20 with the same molarity were made as controls. After co-culturing those at 37 ℃ for 2 h,the free virus and compound were eliminated entirely by washing with PBS and the fresh medium without the test compound was added into each well. After culturing the dish at 37 ℃ for 2 days,the luciferase activity was measured and percentages of inhibition were calculated [8]. 2.2.5. Molecular modeling
HIV-1 gp41 N-peptide bundle data (PDB ID: 2r5d) [14] were obtained from the RCSB Protein Data Bank. Using Molegro Virtual Docker (version 4.3.0),all the molecular modeling calculations were performed. The initial random molecular structures and their geometry were built and optimized by use of ChemOffice 2004. 3. Results and discussion
As shown in Table 1,the D-glucosamine derivative of gossypol (compound 1,IC50 = 1.01 μmol/L,SI = 20.33) not only had better inhibitory activity on HIV-1ⅢB replication in TZM-bl cells,but it also had less cytotoxicity than gossypol. However,compound 2 had evident cytotoxicity on TZM-bl cells,whereas its IC50 was not determined (data no shown). The results implied that acetylation of the D-glucosamine moiety in compound 1 significantly enhanced the cytotoxicity of gossypol. On the other hand, oligopeptide derivatives of gossypol with the COONa group (compounds 3-8) showed more potent activity against HIV-1 and less cytotoxicty with an IC50 from 1.58 to 2.24 μmol/L and SI [23.56-30.54] when compared with gossypol (IC50 = 9.68 μmol/L, SI = 1.01),which supported our previous results [7]. Our previous results suggested that the COONa group might play an important role in mediating the anti-HIV-1 activity of amino acids derivatives of gossypol [7]. Nevertheless,the D-glucosamine derivative of gossypol which lacked the COONa group (compound 1) also exhibited the same potent anti-HIV-1 activity as the oligopeptide derivatives (compounds 3-8). Therefore,compound 1 appeared to be the most interesting antiviral agent among these compounds.
| Table 1 Effects of novel gossypol derivatives on the replication of HIV-1ⅢB in TZM-bl cells and the binding constants (KAs) with HSA. |
The binding constants (KAs) of novel gossypol derivatives with human serum albumin (HSA) were listed in Table 1. Results from Table 1,indicated that these compounds had much lower affinities for HAS than gossypol,whereas these compounds retained better anti-HIV-1 activity than gossypol.
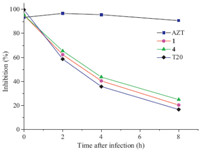
Then,a time-of-addition assay was carried out to investigate the possible target phase of compounds 1 and 4 in the HIV-1 life cycle. From Fig. 1,the time-of-addition assay results suggested that compound 1,compound 4,and T20 inhibited HIV-1 replication when they were added to the cells with virus together,but they showed no inhibitory activity if they were added 2 h or longer after virus was added to the cells. Nevertheless,AZT (3'-azido-3'- deoxythymidine,zidovudine) was still effective in inhibiting HIV-1 replication even when it was added 8 h postinfection. The results showed that compounds 1 and 4 could block the entry of HIV-1ⅢB into target cells,which was similar to T20,likely targeting HIV- 1gp41 as some amino acid derivatives of (-)-gossypol [8].
|
Download:
|
| Fig. 1.Compounds 1 and 4 inhibited HIV-1ⅢB entry. Inhibition of HIV-1ⅢB entry was determined by a time-of-addition assay. | |
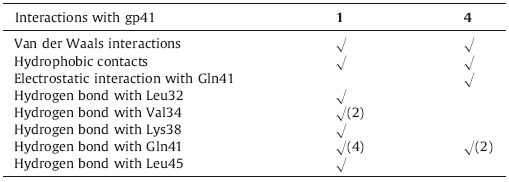
Furthermore,the binding modes of compounds 1 and 4 are shown in Fig. 2. The key interactions between them and gp41 protein residues are summarized in Table 2,which shows that these compounds might deeply dock into the same cavity through Van der Waals interactions,hydrophobic contacts,and hydrogen bonding networks. As shown in Fig. 2,two D-glucosamine moieties of compound 1 are closed to the Lys38 and Gln41,respectively. They are positioned in such a way that these alcoholic hydroxyl groups in the two D-glucosamine moieties of compound 1 can interact with Leu45,Gln41,Lys38,Val34,and Leu32 in the hydrophobic pocket of HIV-1 gp41,which can generate more favorable hydrogen bonding networks and increase affinities with the gp41 protein,whereas the most favorable binding mode of compound 1 is different from those of known HIV-1 gp41 inhibitors [7, 15]. Unlike the binding mode of L-alanine derivative of gossypol [7],one of the negatively charged carboxylic groups of compound 4 is close to Gln41 but not Lys38 in the most favorable binding mode of compound 4 (Fig. 2). Thus,the negatively charged carboxylic group of compound 4 forms an H-bond and a strong electrostatic interaction with Gln41 but not positively charged Lys38. Interestingly,the other negatively charged carboxylic group of compound 4 points toward the outside of the hydrophobic pocket. Therefore,we postulate that the dipeptide chain length of compound 4 can lead to the carboxylic acid group being placed far from the positively charged residues such as Lys38,leading to weaker activity than some amino acid derivatives of gossypol [7, 8].

|
Download:
|
| Fig. 2.Docking conformations of active compound 1 (left) or compound 4 (right) inside the hydrophobic pocket of HIV-1gp41 and residues of the pocket surrounding ligands. | |
| Table 2 The key interactions between some compounds and gp41 protein residues. |
A series of novel gossypol derivatives were synthesized and screened for their in vitro anti-HIV-1 activity. Replacement of the aldehyde groups of gossypol with some water soluble substituents, such as certain oligopeptides and D-glucosamine,not only reduced the cytotoxicity of gossypol derivatives but also enhanced their anti-HIV-1 activity. Compound 1 showed promising anti-HIV-1 activity and appeared to be the most interesting antiviral agent. The results showed that certain oligopeptides and D-glucosamine were also important fragments to prepare gossypol derivatives as HIV-1 entry inhibitors besides certain amino acids.
AcknowledgmentWe gratefully acknowledge the National Natural Science Foundation of China (No. 30770228) for financial support.
| [1] | R. Adams, T.A. Geissman, J.D. Edwards, Gossypol, a pigment of cottonseed, Chem. Rev. 60 (196') 555-574. |
| [2] | R.E. Royer, L.M. Deck, T.J. Vander Jagt, et al., Synthesis and anti-HIV activity of 1,10-dideoxygossypol and related compounds, J. Med. Chem. 38 (1995) 2427-2432. |
| [3] | H. Keshmiri-Neghab, B. Goliaei, Therapeutic potential of gossypol: an overview, Pharm. Biol. 52 (2014) 124-128. |
| [4] | T.S. Lin, R. Schinazi, B.P. Griffith, et al., Selective inhibition of human immunodeficiency virus type 1 replication by the (-) but not the (+) enantiomer of gossypol, Antimicrob. Agents Chemother. 33 (1989) 2149-2151. |
| [5] | P.A. Keller, C. Brich, S.P. Leach, et al., Novel pharmacophore-based methods reveal gossypol as a reverse transcriptase inhibitor, J. Mol. Graph. Model. 21 (2003) 365-373. |
| [6] | T.S. Lin, R.F. Schinazi, J.L. Zhu, et al., Anti-HIV-1 activity and cellular pharmacology of various analogs of gossypol, Biochem. Pharmacol. 46 (1993) 251-255. |
| [7] | J. Yang, F. Zhang, J.R. Li, et al., Synthesis and antiviral activities of novel gossypol derivatives, Bioorg. Med. Chem. Lett. 22 (2012) 1415-1420. |
| [8] | T. An, W.J. Ouyang, W. Pan, et al., Amino acid derivatives of the (-)-enantiomer of gossypol are effective fusion inhibitors of human immunodeficiency virus type 1, Antivir. Res. 94 (2012) 276-287. |
| [9] | K. Liu, H. Lu, L. Hou, et al., Design, synthesis, and biological evaluation of Ncarboxyphenylpyrrole derivatives as potent HIV fusion inhibitors targeting gp41, J. Med. Chem. 51 (2008) 7843-7854. |
| [10] | J. Su, Y. Liu, Z.B. Zheng, et al., Synthesis of aromatic-linked polyamine macrocyclic derivatives as HIV-1 entry inhibitors, Chin. Chem. Lett. 18 (2007) 1166-1168. |
| [11] | J.F. Xu, Z.J. Fang, C.L. Ju, Synthesis of 1, 3, 4, 6-tetra-O-acetyl-β-δ-glucosamine, Chin. J. Synth. Chem. 11 (2003) 379-380, 433. |
| [12] | Analytical data for compounds: 1: Yellow crystal; 92.9% yield, mp 213-214℃(decomposition); IR (KBr, cm-1): vmax 3483, 3361, 2962, 2928, 1616, 1504, 1369, 1311, 1247, 1092, 1038; 1H NMR (400 MHz, DMSO-δ6): δ 13.1896 (broad, 2H, 2×=CH-NH), 9.7048 (broad, 2H, ×=CH-NH), 8.4878 (s, 2H, 2×6-OH), 7.7589 (s, 2H, 2×Glu-OH), 7.4442 (s, 2H, 2×4-H), 5.3526 (s, 2H, 2×Glu-OH), 5.2442 (s, 2H, 2×Glu-OH), 5.2017 (d, 2H, J=7.8 Hz, 2×Glu-C17HN), 5.0785 (s, 2H, 2×Glu-OH), 3.6644-3.3482 (m, 12H, 2 × 4×Glu-CH, 2×Glu-CH2), 3.1835 (sept, 2H, 2×CH(CH3)2), 1.9323 (s, 6H, 2×Ar-CH3), 1.4344 (d, 12H, J=6.3 Hz, 2×CH(CH3)2); 13C NMR (100 MHz, DMSO-δ6): δ 171.9593, 162.3969, 149.5768, 146.3828, 131.0172, 126.8700, 126.2059, 120.0529, 116.4998, 116.0120, 103.1450, 90.6044, 72.3004, 71.5499, 70.3252, 60.7674, 55.7574, 26.4723, 20.3102, 20.3102, 20.1543; MS (ESI+): m/z 841.8 [M+H]+. 2: Yellow crystal; 92.6% yield, mp 177-178℃; IR (KBr, cm-1):vmax 3485, 2961, 1756, 1610, 1542, 1490, 1418, 1369, 1321, 1216, 1077, 1038; 1H NMR (300 MHz, CDCl3):δ 13.505 (s, 2H, ×=CH-NH), 9.581 (d, 2H, J=9.3 Hz, ×=CH-NH), 7.755 (s, 2H, 2×-OH), 7.599 (s, 2H, 2×4-H), 5.854 (d, 2H, J=7.8 Hz, 2×Glu-C17HN), 5.609 (s, 2H, 2×1-OH), 5.428-3.702(m, 12H, 2×4×Glu-CH, 2×Glu-CH2), 3.592 (sept, 2H, 2×CH(CH3)2), 2.073(s, 24H, 2 × 4×COCH3), 1.958 (s, 6H, 2×Ar-CH3), 1.515 (d, 12H, J=6.3 Hz, 2×CH(CH3)2); 13C NMR(75 MHz, CDCl3):δ 173.64, 170.57, 169.88, 169.63, 168.71, 162.49, 149.16, 146.80, 132.36, 129.49, 128.40, 118.53, 115.98, 114.22, 104.15, 92.07, 72.68, 72.33, 67.71, 63.99, 61.48, 27.43, 20.79, 20.65, 20.59, 20.46, 20.24, 20.19, 19.96; MS (ESI+): m/z 1176.1 [M-H]-. 3: Yellow crystal; 88.2% yield, mp 210℃(decomposition); IR (KBr, cm-1):vmax 3483, 3377, 2963, 2871, 1616, 1520, 1494, 1401, 1308, 1246, 1168, 1021; 1HNMR(400 MHz, DMSOd6):δ13.2702 (s, 2H, 2×=CH-NH), 9.6983 (s, 2H, ×=CH-NH), 8.3898 (s, 2H, 2×-OH), 8.1827 (m, 2H, 2×N19-H), 7.3605 (s, 2H, 2×4-H), 4.4452 (m, 4H, 2×20-H), 3.6750 (m, 2H, 2×C17HN), 3.5397 (sept, 2H, 2×CH(CH3)2), 1.9473 (d, 6H, J=5.5 Hz, 2×17-CH3), 1.8841 (s, 6H, 2×Ar-CH3), 1.4247 (d, 12H, J=6.3 Hz, 2×CH(CH3)2); 13C NMR (100 MHz, DMSO-δ6):δ177.8820, 177.4359, 175.0255, 157.9823, 151.4497, 145.2643, 132.0330, 128.6840, 127.1184, 121.3668, 117.6309, 116.2757, 103.4103, 61.2516, 48.2834, 26.8479, 25.5420, 25.5420, 25.2018, 23.7551; MS (ESI+): m/z 773.8 [[M-2Na+H]-2Na+H]-. 4: Yellow crystal; 85.3% yield, mp 237℃(decomposition); IR (KBr, cm-1):vmax 3483, 3371, 2973, 2932, 2871, 1614, 1520, 1453, 1407, 1365, 1309, 1244, 1168, 1056; 1H NMR (400 MHz, DMSO-δ6):δ13.2743 (s, 2H,5CH-NH), 10.0064 (s, 2H, ×=CH-NH), 8.2501 (s, 2H, 2×=5OH), 8.1609 (d, 2H, J=6.5 Hz, 2×N19-H), 7.3257 (s, 2H, 2×4-H), 4.4312 (m, 2H, 2×20-H), 3.9447 (m, 2H, 2×C17HN), 3.6738 (sept, 2H, 2×CH(CH3)2), 1.9384 (d, 6H, J=5.5 Hz, 2×20-CH3), 1.8869 (s, 6H, 2×Ar-CH3), 1.4259 (d, 12H, J=6.2 Hz, 2×CH(CH3)2), 1.1897 (d, 6H, J=5.5 Hz, 2×17-CH3); 13C NMR (100 MHz, DMSOd6):δ174.9651, 172.5518, 169.2470, 160.3944, 150.9108, 146.1267, 131.1563, 126.7315, 126.5257, 121.0936, 116.4858, 116.0166, 103.7602, 55.9979, 49.4534, 26.4918, 20.3004, 20.3004, 20.0538, 18.6808, 18.5059; MS (ESI+): m/z 801.9[M-2Na+H]-. 5: Yellow crystal; 90.8% yield, mp 255℃(decomposition); IR (KBr, cm-1):vmax 3483, 3356, 2968, 2871, 1609, 1520, 1453, 1405, 1364, 1313, 1242. 1H NMR (400 MHz, DMSO- |
| [13] | J. Yang, Z.H. Jing, J.J. Jie, P. Guo, Fluorescence spectroscopy study on the interaction between gossypol and bovine serum albumin, J. Mol. Struct. 920 (2009) 227-230. |
| [14] | B.D. Welch, A.P. VanDemark, A. Heroux, C.P. Hill, M.S. Kay, Potent D-peptide inhibitors of HIV-1 entry, Proc. Natl. Acad. Sci. U. S. A. 104 (2007) 16828-16833. |
| [15] | G.Y. Zhou, S.D. Chu, Discovery of small molecule fusion inhibitors targeting HIV-1 gp41, Curr. Pharm. Des. 19 (2013) 1818-1826. |