b University of Chinese Academy of Sciences, Beijing 100049, China
Type 2 diabetes mellitus is a metabolic disorder and chronic disease that is characterized by high blood glucose in the context of insulin resistance and relative insulin deficiency. Diabetic patients normally suffer from complications,such as nephropathy, neuropathy,cataract formation,retinopathy,atherosclerosis, cardiovascular disease,and cerebrovascular pathologies [1, 2, 3]. Long-term secondary complications are the main cause of morbidity and mortality of diabetic patients [4]. Thus preventing, or delaying the onset and progression of diabetic complications has become one of the key issues in medical research [5, 6]. Several mechanisms have been proposed for these complications,some evidence suggests that the polyol pathway,formation of advanced glycation end-products (AGEs),and oxidative stress are associated with the etiology of these chronic diseases [7, 8]. Aldose reductase (AR,EC 1.1.1.21),which is the key rate-limiting enzyme in the polyol pathway,catalyzes the reduction of glucose to sorbitol in the presence of NADPH [9]. The development of diabetic complications could be controlled by retarding AR activity and also by inhibiting the formation of AGEs [7].
At present,available drugs for type 2 diabetes mellitus show a number of limitations,such as side effects and high rates of secondary failure. The diabetes control and complications trial had demonstrated that even an optimal control of blood glucose could not prevent complications,suggesting that alternative treatment strategies are needed [10]. As a complementary/alternative approach,medicinal herbs with anti-hyperglycemic activities are increasingly administered to diabetic patients by healthcare professionals. Furthermore,traditional Chinese medicines (TCMs), with a long history and unique theory system and a variety of herb remedies,have been attracting more and more attention for their complementary therapeutic effects to western medicines [11, 12, 13].
The aim of this investigation was to evaluate the activities of eleven natural product extracts to inhibit AR and AGEs formation. An ultrahigh performance liquid chromatography and tandem mass spectrometry (UPLC-MS/MS) method was used to determine the AR inhibitory activities of herbal extracts. Additionally,the fluorometric method was applied to evaluate the inhibitory activities of herbal extracts on AGEs formation in glycation model reactions. The results obtained in this study could provide a reference for clinical studies in managing diabetic complications. 2. Experimental 2.1. Reagents and chemicals
Sorbitol,xylitol,ammonium acetate (NH4Ac) and DL-glyceraldehyde were purchased from Sigma Chem. Co.,(St. Louis,MO, USA). β-NADPH was obtained from F. Hoffmann-La Roche Ltd. (Basel,Switzerland). β-Mercaptoethanol,NaN3 and bovine serum albumin (BSA) were purchased from Dingguo Biotec. Co.,(Beijing, China). Amino guanidine (hemi-sulfate salt) was supplied from Aladdin (Shanghai,China). Epalrestat was obtained from the Yangtze River Pharmaceutical Group (Taizhou,China). Methanol, HPLC grade,was obtained from Fisher Scientific (Fair Lawn,NJ, USA). Deionized water was prepared using a Milli-Q water purification apparatus (Bedford,MA,USA). All other chemicals and reagents were of analytical grade. 2.2. Preparation of herbal extracts
The natural products were purchased from Tongrentang Pharmacy (Changchun,China) and authenticated by Professor Shumin Wang (Changchun University of Traditional Chinese Medicine,China). The plant samples were ground into uniform powder using a high-speed grinder. The powder,after passing a 100 mesh sieve,was weighed accurately,and then a 60% ethanol extraction was carried out at 30°C for 40 min using ultrasoundassisted extraction process. These mixtures were centrifuged at 4000 rpm for 10 min and the supernatants were filtered through a 0.22 μm membrane filter. Afterward,the filtrates were quantified with the same solvent and the obtained stock solutions of 200 mg/mL were stored at 4°C. 2.3. Preparation of AR
The preparation of partially purified AR was performed as previously described [14, 15] but with minor modifications. The crude AR solution was obtained from bovine lenses by using ammonium sulphate fractionated precipitation (40%-75%) and ultra-filtration method. The crude enzyme was then verified by using SDS-polyacrylamide gel electrophoresis. Afterwards,the activity of AR was evaluated based on DL-glyceraldehyde as a substrate,according to the previous study [15]. The change in the absorbance at 340 nm due to NADPH oxidation was followed in a Tecan GENios Microplate Reader (Ma¨nnedorf,Switzerland). Assays were carried out at 25°C with an appropriated blank subtracted from each reaction to correct for nonspecific oxidation of NADPH. 2.4. AR inhibitory activity
Since DL-glyceraldehyde was used as the surrogate substrate of glucose and the traditional spectrometric method for measuring the change in absorbance at 340 nm of NADPH was an indirect manner for the screening of AR inhibitors,the likelihood of falsepositive results increased because NADPH was extremely unstable in acidic medium. In view of these facts,an UPLC-MS/MS method was developed to evaluate the activities of the compounds in this experiment. Glucose,which was reduced to sorbitol in the polyol pathway,was chosen as the substrate of the enzymatic reaction in vitro. Then sorbitol was directly detected using multiple reaction monitoring (MRM) mode and the AR inhibitory activities of natural products was evaluated. Two precursor/product ion transitions were elected in creating the MS/MS method to collect sufficient data for the integrative research. The ion transition with the highest sensitivity was chosen for the quantitative analysis; while a second ion transition was selected as the qualitative ion pair for the confirmatory analysis. The ion transitions with appropriate instrumental parameters for the MRM detection are listed in Table 1.
| Table 1 Ion transitions and instrumental parameters of sorbitol and xylitol for MRM mode. |
The UPLC-MS/MS analyses were performed using ACQUITYTM UPLC system combined with a XevoTM TQ mass spectrometer (Waters,Milford,MA,USA). A Waters UPLC® BEH Shield RP 18 column (100 mm × 2.1 mm,i.d.,1.7 μm,Milford,MA,USA) was applied in the chromatographic separation. The binary mobile phase consisted of methanol-water (20:80,v/v),the elution was performed at a flow rate of 0.2 mL/min,with 3 mL of sample was injected. The column temperature was set at 35°C,while the temperature of sample manager was maintained at 4°C. The column outlet of the UPLC system was connected via capillary to the electrospray ionization (ESI) source of the mass spectrometer. The spray voltage was set at 3.0 kV in positive ion mode. The flow rates of cone gas and desolvation gas were set to 60 and 800 L/h,respectively. The ion source temperature and desolvation temperature were maintained at 150 and 350°C,respectively. The data were acquired via MassLynx4.1 software (Waters),and the MassLynx4.1 with TargetLynx was applied to data processing. 2.5. AGEs formation inhibitory activity
The AGEs formation inhibitory effect of herbal extracts was performed as previously described [16],but with some modifications. Because 0.02% sodium azide could prevent the growth of microorganisms and not interact with protein,BSA was dissolved in the phosphate buffer with 0.02% sodium azide to prevent degradation. Fructose and glucose were prepared together in 0.02% sodium azide,while the herbal extracts were freshly prepared. Briefly,the reaction mixture contained 50 μmol/L phosphate buffer (pH 7.4),10 mg/mL BSA,200 μmol/L glucose,200 μmol/L fructose,and 10 mg/mL extracts (diluted with phosphate buffer). The reaction solution (1 mL) was incubated at 37°C for 14 days, while the negative control was kept at 4°C. Amino guanidine (2 mg/mL) was used as a positive control in the AGEs inhibition assay. All incubations were done in quadruplicate. The fluorescence intensity of the sample was determined with excitation and emission wavelengths at 370 and 440 nm,respectively,on a Tecan GENios Microplate Reader (Ma¨nnedorf,Switzerland). The inhibition percentage (%) was calculated according to the following formula:

Hence,F1 and F2 are the fluorescence intensities at 440 nm of analytes in the presence,or absence of an analyte,while F0 is the fluorescence intensity of the blank sample. 2.6. Method validation
The suitability of the UPLC-MS/MS method was properly verified in order to ensure the results obtained were reliable. Method validation was implemented by evaluated performance characteristics in terms of linear range,accuracy,precision,matrix effects and analytical limits including method limit of quantification (LOQ). 3. Results and discussion 3.1. Verification of AR
Coomassie Blue staining clearly demonstrated the abundance of the protein with an apparent molecular size of 36 kDa in crude AR. According to a previous report [17],one unit of enzyme activity was defined as the amount of the enzyme that catalyzes the conversion of 1 μmol of NADPH per minute under the assay conditions. The activity unit of crude enzyme was 14.3,therefore the crude AR could be used in our experiment. 3.2. AR inhibition activity
Complications that develop during a diabetic state can be retarded by inhibiting the key enzymes involved in the polyol pathway,i.e.,aldose reductase,which can in turn help prevent the formation of AGEs. AR inhibitors have been shown to be able to delay or substantially prevent the chronic complications of diabetes,such as retinopathy,cataract,neuropathy,nephropathy, myocardial ischemic injury and atherosclerosis [1, 2, 3, 18]. In recent years,the UPLC-MS/MS approach fulfills key requirements in terms of rapidity,sensitivity,selectivity and peak-assignment certainty for the analysis of complex matrices with low analyte concentrations,so UPLC-MS/MS has become one of optional techniques in the screening of inhibitors. Additionally,the MRM function,which is a specific and sensitive quantitative method of MS/MS analysis,could be used to monitor multiple target ions of each analyte simultaneously [19, 20]. In our study,UPLC-MS/MS was applied to screening AR inhibitors from herbal extracts. The reaction solution consisted of 100 μmol/L ammonium acetate buffer (pH 6.2),5 mmol/L β-mercaptoethanol,30 μL AR enzyme, 0.45 mmol/L NADPH,20 μL extract samples,and 5 mmol/L glucose as the substrate. The reaction system of a total volume of 200 mL was incubated at 37°C for 25 min,and then terminated by adding 800 mL of methanol. Then,xylitol as the internal standard was added to the reaction system with a final concentration of 1 μmol/ L. The inhibiting capacity of analytes was evaluated according to the concentration of sorbitol in the enzymatic reaction system using the UPLC-MS/MS analysis. Epalrestat was used as positive control,while negative control was the assay performed without the sample. All the screening assays were performed in triplicate. In this study,the AR inhibition (%) of herbal extracts was calculated as follows:

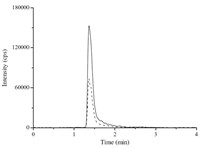
All the herbals were classified into four classes depending on the main ingredients: the herbs rich in flavonoids including Flos Sophorae Immaturus,Radix Scutellariae and Folium Acanthopanacis Senticosi; the herbs rich in glycosides and iridoids including Cortex Cinnamomi,Fructus Corni,Fructus Schisandrae Chinensis,Radix Rehmanniae Praeparata and Radix Paeoniae Rubra; herbs rich in saponins including Radix Glycyrrhizae and Rhizoma Anemarrhena; while anthraquinones were the main ingredients of Radix Salviae Miltiorrhizae. Fig. 1 showed the MRM chromatograms of sorbitol in screening AR inhibitors from herbal extracts. The AR inhibitory activities of herbal extracts are shown in Table 2. The flavonoidrich herbal extracts were found to be more effective than other herbs in inhibiting AR activity,respectively,thus suggesting their potential use in preventing and treating diabetic complications, while the glycosides and iridoids class generally showed good AR inhibitory activities. On the contrary,the herbal extracts rich in saponins and anthraquinones showed weak inhibitory effects on AR activity. Epalrestat,a carboxylic acid derivative,is currently marketed for use in the treatment of diabetic neuropathy in Japan [21]. Flavonoids from plants have been reported to have the potential to be developed as therapeutic agents for diabetic complications and oxidative stress-related diseases due to their AR inhibition activity [20]. At present,a great deal of AR inhibitors obtained from natural sources,such as flavonoids,stilbenes, coumarins,monoterpenes and related aromatic compounds,have been reported [22, 23, 24]. Based on the above analysis and the results, the AR inhibitory activity of herbal extracts could be due to their particular bioactive components,such as flavonoids,glycosides and iridoids.
|
Download:
|
| Fig. 1.The MRM chromatograms of sorbitol in screening inhibitory effect of herbal extracts on AR activity (solid line: experiment without AR inhibitors,dash line: experiment with AR inhibitors). | |
Non-enzymatic glycation reaction of protein is a process in which the carbonyl groups of reducing sugars and the free amino groups of protein react to produce AGEs [25]. Endogenous formation of AGEs is known to contribute to the progression of pathogenesis in conditions associated with diabetic complications and ageing. Recent attention has focused on the benefits of medicinal plants with both antiglycation and antioxidant properties [26, 27].
In our research,the inhibitory effect of herbal extracts on AGEs formation was calculated using Eq. (1) and amino guanidine was used as a positive control in this AGEs inhibition assay. The results summarized in Table 2 indicated that the inhibitory strengths of herbal extracts were different from each other. It showed that the AGEs formation inhibitory activity of Flos Sophorae Immaturus, Radix Scutellariae and Rhizoma Anemarrhenae were better than others in the BSA/glucose (fructose) system by fluorescene analysis. The major constituents of Flos Sophorae Immaturus and Radix Scutellariae are rutin and baicalin,respectively,while the major component of Rhizoma Anemarrhenae is timosaponin. It has also been reported that rutin and baicalin had remarkable antioxidant activities [28, 29, 30]. So,the inhibitory effect on the non-enzymatic protein glycation of Flos Sophorae Immaturus and Radix Scutellariae may be associated with their antioxidant activities. The previous researches showed that the potential ingredients would affect greatly the anti-glycation activity of natural products,and the amount of phenolic and flavonoid in herbal extracts highly correlated with their activity [31]. In view of the above-mentioned,the capacity of herbal extracts in inhibiting the formation of fluorescent AGEs may be depended upon their bioactive ingredients.
| Table 2 The anti-hyperglycemia capacities of eleven kinds of herbal extracts. |
The linear calibration curve was not forced though the origin and was corrected by the weighting coefficient,1/x. Linear regression equation of sorbitol was y = 0.831363x + 0.00686187 in the concentration range of 0.1-20 μmol/L and the correlation coefficient (r2) was 0.999. The LOQ was appropriate for quantitative detection of analytes in the enzymatic activity studies. The intra-day and inter-day precision and accuracy were investigated at three levels (five parallels of each concentration). Relative error (RE) and relative standard deviation (RSD) were used to express the accuracy and precision,respectively. The precision and accuracy results are shown in Table 3,which met the requirements of an assay. Furthermore,the matrix effect was evaluated by comparing the response of an analyte in neat solution to the response of the analyte spiked into a blank matrix sample that had been carried through the sample preparation process.
| Table 3 Precision and accuracy of sorbitol of UPLC-MS/MS method (n = 5). |
If the AGEs formation and AR inhibition ratios of some herbal extracts were higher than that of others at the same concentration in vitro,the herbal extracts were considered possessing the potential to be developed as a therapeutic agent for diabetic complications and oxidative stress-related diseases. In this research,the inhibitory activities of eleven herbal extracts on AR activity and AGEs formation were estimated using the UPLC- MS/MS method and fluorescent spectrometry,respectively. According to our results,Flos Sophorae Immaturus and Radix Scutellariae seemed to be more effective in inhibiting AGEs formation and AR activity compared with other screened herbs. The major ingredients of Flos Sophorae Immaturus and Radix Scutellariae were flavonoids which had exhibited potential efficacy in diabetic complications and oxidative stress-related diseases; and in our experiment,the AR inhibition activities of them were higher than other herbal extracts and were consistent with the preceding statements. The inhibiting capacities of herbal extracts against AR activity and AGEs formation may be correlated with the bioactive components of the herbal extracts. In this study,we have estimated the potential anti-hyperglycemic bioactivities of eleven herbal extracts in vitro,which could provide a reference for the further study in the prevention and treatment of diabetic complications. 4. Conclusion
The inhibitors of AR and AGEs formation have been considered to apply to the potential treatment of diabetic complications. In the present work,the inhibitory abilities of eleven herbal extracts on AR activity and AGEs formation were estimated. The results showed that the inhibitory activities of Flos Sophorae Immaturus and Radix Scutellariae were stronger than other herbal extracts. Since the inhibitory abilities of natural products against AR activity and AGEs formation may be related to their bioactive components, these results provide a reference for the further study of the application of natural products and their bioactive constituents in the management of diabetic complications. Acknowledgments
This research was financially supported by the National Natural Science Foundation of China (No. 81373952) and the Innovation Method Fund of China (No. 2012IM030600).
| [1] | D.J. Porte, M.W. Schwartz, Diabetes complications: why is glucose potentially toxic? Science 272 (1996) 699-700. |
| [2] | I.M. Stratton, A.I. Adler, H.A. Neil, et al., Association of glycaemia with macrovascular and microvascular complications of type 2 diabetes (UKPDS 35): prospective observational study, Br. Med. J. 321 (2000) 405-412. |
| [3] | R. Ramasamy, I.J. Goldberg, Aldose reductase and cardiovascular diseases, creating human-like diabetic complications in an experimental model, Circ. Res. 106 (2010) 1449-1458. |
| [4] | M. Brownlee, Biochemistry and molecular cell biology of diabetic complications, Nature 414 (2001) 813-820. |
| [5] | J.T. Xie, A. Wang, S. Mehendale, et al., Anti-diabetic effects of Gymnema yunnanense extract, Pharmacol. Res. 47 (2003) 323-329. |
| [6] | R.A. DeFronzo, Pharmacologic therapy for type 2 diabetes mellitus, Ann. Intern. Med. 131 (1999) 281-303. |
| [7] | K. Tsuji-Naito, H. Saeki, M. Hamano, Inhibitory effects of Chrysanthemum species extracts on formation of advanced glycation end products, Food Chem. 116 (2009) 854-859. |
| [8] | O. El-Kabbani, F. Ruiz, C. Darmanin, R.P. Chung, Aldose reductase structures: implications for mechanism and inhibition, Cell. Mol. Life Sci. 61 (2004) 750-762. |
| [9] | D.K. Wilson, K.M. Bohren, K.H. Gabbay, F.A. Quiocho, An unlikely sugar substrate site in the 1.65Åstructure of the human aldose reductase holoenzyme implicated in diabetic complications, Science 257 (1992) 81-84. |
| [10] | H. Pareek, S. Sharma, B.S. Khajja, K. Jain, G.C. Jain, Evaluation of hypoglycemic and anti-hyperglycemic potential of Tridax procumbens (Linn.), BMC Complem. Alter. Med. 9 (2009) 48-54. |
| [11] | D. Normile, The new face of traditional Chinese medicine, Science 299 (2003) 188-190. |
| [12] | J. Yin, H. Zhang, J. Ye, Traditional Chinese medicine in treatment of metabolic syndrome, Endocr. Metab. Immune. Disord. Drug Targets. 8 (2008) 99-111. |
| [13] | L. Liu, J.A. Duan, Y. Tang, et al., Taoren-Honghua herb pair and its main components promoting blood circulation through influencing on hemorheology, plasma coagulation and platelet aggregation, J. Ethnopharmacol. 139 (2012) 381-387. |
| [14] | S. Hayman, J.H. Kinoshita, Isolation and properties of lens aldose reductase, J. Biol. Chem. 240 (1965) 877-882. |
| [15] | C. Nishimura, T. Yamaoka, M. Mizutani, et al., Purification and characterization of the recombinant human aldose reductase expressed in baculovirus system, Biochim. Biophys. Acta 1078 (1991) 171-178. |
| [16] | J.A. Vinson, T.B. Howard III, Inhibition of protein glycation and advanced glycation end products by ascorbic acid and other vitamins and nutrients, J. Nutr. Biochem. 7 (1996) 659-663. |
| [17] | J.Y. Wang, S.G. Zhu, C.F. Xu, Biochemistry, 3rd ed., Higher Education Press, Beijing, 2007. |
| [18] | Y. Kawanishi, S. Noparatanawong, S. Kamohara, M. Nakano, Antioxidants supplementation prevents exercise induced oxidative damage in healthy subjects, J. Am. Diet. Assoc. 103 (2003) 36. |
| [19] | C.S. Wu, Y. Jin, J.L. Zhang, Y. Ren, Z.X. Jia, Simultaneous determination of seven prohibited substances in cosmetic products by liquid chromatography-tandem mass spectrometry, Chin. Chem. Lett. 24 (2013) 509-511. |
| [20] | S. Liu, J.P. Xing, Z. Zheng, et al., Ultrahigh performance liquid chromatography-triple quadrupole mass spectrometry inhibitors fishing assay: a novel method for simultaneously screening of xanthine oxidase inhibitor and superoxide anion scavenger in a single analysis, Anal. Chim. Acta 715 (2012) 64-70. |
| [21] | N. Hotta, Y. Akanuma, R. Kawamori, et al., Long-term clinical effects of epalrestat, an aldose reductase inhibitor, on diabetic peripheral neuropathy: the 3-year, multicenter, comparative aldose reductase inhibitor-diabetes complications trial, Diabetes Care 29 (2006) 1538-1544. |
| [22] | H.A. Jung, M.D. Islam, Y.S. Kwon, et al., Extraction and identification of three major aldose reductase inhibitors from Artemisia montana, Food Chem. Toxicol. 49 (2011) 376-384. |
| [23] | K. Kawanishi, H. Ueda, M. Moriyasu, Aldose reductase inhibitors from the nature, Curr. Med. Chem. 10 (2003) 1353-1374. |
| [24] | J.Á. de la Fuente, S. Manzanaro, Aldose reductase inhibitors from natural sources, Nat. Prod. Rep. 20 (2003) 243-251. |
| [25] | S. Katayama, Y. Haga, H. Saeki, Loss of filament-forming ability of myosin by nonenzymatic glycosylation and its molecular mechanism, FEBS Lett. 575 (2004) 9-13. |
| [26] | T. Manaharan, L.L. Teng, D. Appleton, et al., Antioxidant and antiglycemic potential of Peltophorum pterocarpum plant parts, Food Chem. 129 (2011) 1355-1361. |
| [27] | X. Wang, L.S. Zhang, L.L. Dong, Inhibitory effect of polysaccharides from pumpkin on advanced glycation end-products formation and aldose reductase activity, Food Chem. 130 (2012) 821-825. |
| [28] | J.X. Yang, J. Guo, J.F. Yuan, In vitro antioxidant properties of rutin, LWT -Food Sci. Technol. 41 (2008) 1060-1066. |
| [29] | Z.H. Gao, H.B. Xu, X.J. Chen, H. Chen, Antioxidant status and mineral contents in tissues of rutin and baicalin fed rats, Life Sci. 73 (2003) 1599-1607. |
| [30] | H.J. Heo, D.O. Kim, S.J. Choi, D.H. Shin, C.Y. Lee, Potent inhibitory effect of flavonoids in Scutellaria baicalensis on amyloid beta protein-induced neurotoxicity, J. Agric. Food Chem. 52 (2004) 4128-4132. |
| [31] | S.C. Ho, S.P. Wu, S.M. Lin, Y.L. Tang, Comparison of anti-glycation capacities of several herbal infusions with that of green tea, Food Chem. 122 (2010) 768-774. |







