b Department of Entomology, College of Agriculture and Biotechnology, China Agricultural University, Beijing 100193, China
Neonicotinoid insecticides (NNs),exemplified by imidacloprid, have agonistic effects on the insect nicotinic acetylcholine receptor (nAChR) [1, 2],which makes this kind of insecticide particularly effective in controlling sap-feeding pests and relatively safe toward mammals [3, 4, 5, 6]. These NNs are widespread and account for onefifth of the global insecticide market [7, 8, 9]. However,during the past decade,increases in resistance and cross resistance were observed in a range of species after their frequent applications in the field [10, 11, 12, 13]. Further,it was reported that NNs have toxicity towards honey bees,which also limited their applications [14]. Hence,it is necessary to design and screen novel insecticidal lead compounds with low resistance and high safety.
Most structure optimizations of NNs are based on cyclic neonicotinoid insecticides [15, 16, 17, 18],but few studies have been focused on the structural modification of acyclic NNs,such as clothianidin. In addition,diacylhydrazine compounds have been widely used as one of the most important insect growth regulators [19, 20],and the hydrazide group has also been proved to be a useful section in the scaffold of active insecticides [21, 22]. Encouraged by this,we hereby introduced a new structure developing strategy,using clothianidin as the lead compound, and a series of novel neonicotinoid derivatives similar to diacylhydrazine were designed by introducing a hydrazide group into clothianidin. Their insecticidal activity against soybean aphids (Aphis glycines) was evaluated at different concentrations. Furthermore,the interactions between these new compounds and nAChR were also investigated by molecular docking. 2. Experimental
Melting points of all compounds were determined on an X-5 binocular microscope (Fukai Instrument Co.,Beijing,China),and were not corrected. 1H NMR spectra were recorded on Bruker AM-300 (300 MHz) spectrometer with DMSO-d6 as the solvent and TMS as the internal standard. Chemical shifts are reported ind (parts per million) values. High resolution mass spectrometry (HRMS) data were obtained on an FTICR-MS Varian 7.0T FTICR-MS instrument. All the reagents were obtained commercially and used after further purification. Column chromatography purification was carried out by using silica gel. 2.1. General procedure for synthesis of compounds 5a-5l
A solution of3(10.0 mmol) and hydrazine hydrate (12.0 mmol) in methanol (20 mL) was refluxed for 0.5 h. Then the mixture was concentrated and the residue was purified by recrystallization with ethanol to afford the pure compound 4. Then a solution of 4 (5.0 mmol),DMAP (0.25 mmol) and TEA (7.5 mmol) in dichloromethane (15 mL) was cooled to -5 to 0°C,and acyl chloride (7.5 mmol) was added dropwise. After that,the mixture was stirred at room temperature for 6 h. After being quenched by water (5 mL),the mixture was filtered,and the precipitate was washed with dichloromethane then purified by recrystallization with ethanol to afford the pure products5a-5l. 2.2. Insecticidal test for soybean aphids (A. glycines)
The activities of the insecticidal compounds against soybean aphids were tested by the leaf-dip method. Horsebean plant leaves with 40-60 apterous adults were dipped in diluted solutions of the chemicals containing Triton X-100 (0.05 mg L-1) for 5 s,the excess dilution was sucked out with filter paper,and the burgeons were placed in the conditioned room (25±1°C,50% RH). Water containing Triton X-100 (0.05 mg L-1) was used as a control,and clothianidin was used as positive control at the same time. The mortality rates were evaluated 48 h after treatment. Each treatment had three repetitions and the data was subjected to probit analysis [23]. 2.3. Experimental protocol of docking study
The highly active compound 5g was chosen for investigation of the ligand-protein interactions in detail with clothianidin as a contrast,and a Surflex-Dock was used to carry out the molecular modelling study. The crystal structure of theAplysia californica acetylcholine binding protein (Ac-AChBP) complexed with thiacloprid (PDB: 3C84) was used as the template to construct the models [24, 25]. The receptor was prepared for docking by the addition of hydrogen atoms and the removal of cocrystallized molecules. The low energy conformation of each neonicotinoid analogue was optimized by a MMFF94 force field and MMFF94 charges as an initial docking conformation. 3. Results and discussion
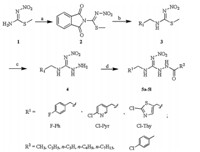
The synthetic route of the target compounds (5a-5l) was summarized in Scheme 1. The starting materialS-methyl-N-nitroisothiourea 1 was prepared by the literature method [26]. Compound 2 was obtained in high yield by reaction of 1 with phthaloyl dichloride at 0°C to room temperature. Then it was reacted with benzylamine and purified by alcohol to afford 3.Compound 4 was produced by hydrazinolysis of 3 with hydrazine hydrate. The target compounds 5a-5l were obtained by reaction of 4 with acyl chloride in moderate yields.
|
Download:
|
| Scheme 1. Synthetic route for neonicotinoids containing hydrazide group. Reagents and conditions: (a) phthaloyl dichloride (1.5 equiv.),pyridine,0°C for 1 h; (b) benzylamine (1.2 equiv.),CH2Cl2,0°C for 1 h,r.t. for 3 h; (c) hydrazine hydrate (1.2 equiv.),CH3OH,reflux for 0.5-1 h; (d) acyl chloride (1.5 equiv.),Na2CO3(1.5 equiv.),DMAP (0.5 equiv.),CH2Cl2,0°C for 0.5 h,r.t. for 6-8 h. | |
The structures of the target compounds 5a-5l were fully confirmed by their melting points, 1HNMR,IR,andHRMS(ESI). Analytical data of compounds 5a-5l can be found in Supporting information. In the 1H NMR spectra,methylene connected to an aromatic ring was observed at &delat;4.34-4.45. Furthermore,three NH protons were clearly detected at &delat; 8.30-8.36,9.91-10.10 and 10.20-10.25 respectively. In the IR spectra,strong absorptions at 3200-3400 cm-1 were detected,due to the secondary amino groups. A strong absorption at about 1700 cm-1 was detected because of carbonyl group. The HRMS (ESI) spectral data of all compounds were in good agreement with theoretical data.
The results of insecticidal activity against soybean aphids (A. glycines) of all target compounds were listed in Table 1,in which some compounds,5b and 5g,showed similar insecticidal activity of 91.5%,94.5% respectively to that of the positive control clothianidin at 500 mg L-1. In addition,5b and 5g exhibited good insecticidal activity of 82.2% and 83.9% respectively at 100 mg L-1. However,5b and 5g showed less activity than clothianidin at 20 mg L-1. The results indicated that a heterocycle substituent, such as pyridyl or thiazolyl,was more beneficial to insecticidal activity than a phenyl substituent. Besides,substituent R2 also played an important role in insecticidal activity. Increasing the number of carbon atoms or the introduction of a phenyl group led to a decline in bioactivity (Fig. 1). A small sized group is conducive to bioactivity,e.g.,5band5ghave better activity than 5f,5g and 5l.
| Table 1 Insecticidal activity of target compounds 5a-5l against soybean aphid (Aphis glycines). |

|
Download:
|
| Fig. 1. Effect of the number of carbon atoms on the insecticidal activity. | |
To further understand the activity differences between the analogues and clothianidin,binding site interactions of 5g and clothianidin were simulated withAc-AChBP based on the structure cocrystallized with bound thiacloprid (3C84). Fig. 2a showed,in compliance with neonicotinoids’ binding mode,the hydrogen bond force and the cation-pinteraction were found between clothianidin and AChBP. Compound 5g showed a similar binding mode with clothianidin (Fig. 2b). However,the introduction of hydrazide into 5g extended the centroid distance between the benzene ring in Tyr188 and N atom of imino group in ligand to 3.18 Å ,which is longer than the 2.84 Å of clothianidin. The longer distance decreased the cation-pinteraction with AChBP. Since cation-pinteraction was confirmed to play an important role in neonicotinoids’ insecticidal activities [27],this decreasing of the cation-pinteraction might lead to weaker insecticidal activity of the analogues than clothianidin.

|
Download:
|
| Fig. 2. The interactional model between ligands andAc-AChBP. (a) The binding interactions between clothianidin and the active site residues. (b) The binding interactions between 5g and the active site residues. Key H-bonds and cation-π are indicated by black dotted lines. | |
In summary,a series of novel clothianidin analogues were designed and synthesized by introducing a hydrazide group with different alkyl and phenyl substituents. The bioassay results indicated that some target compounds,5b and 5g,had good insecticidal activity at 100 mg L-1,but still lower than clothianidin. Molecular docking with receptorAc-AChBP revealed the reason of different activity between the title analogues and clothianidin. These results were worthy for further study in new pesticide discovery. Acknowledgment
This work was financially supported by the National Basic Research Program of China (No. 2010CB126104). Appendix A. Supplementary data Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.03.026.
| [1] | J.E.C. Jepson, L.A. Brown, D.B. Sattelle, The actions of the neonicotinoid imidacloprid on cholinergic neurons of Drosophila melanogaster, Invert. Neurosci. 6 (2006) 33-40. |
| [2] | S. Kagabu, R. Ishihara, Y. Hieda, K. Nishimura, Y. Naruse, Insecticidal and neuroblocking potencies of variants of the imidazolidine moiety of imidacloprid-related neonicotinoids and the relationship topartition coefficient and charge density on the pharmacophore, J. Agric. Food Chem. 55 (2007) 812-818. |
| [3] | K. Kiriyama, K. Nishimura, Structural effects of dinotefuran and analogues in insecticidal and neural activities, Pest Manage. Sci. 58 (2002) 669-676. |
| [4] | M. Tomizawa, D.L. Lee, J.E. Casida, Neonicotinoid insecticides: molecular features conferring selectivity for insect versus mammalian nicotinic receptors, J. Agric. Food Chem. 48 (2000) 6016-6024. |
| [5] | S.J. Lee, M. Tomizawa, J.E. Casida, Nereistoxin and cartap neurotoxicity attributable to direct block of the insect nicotinic receptor/channel, J. Agric. Food Chem. 51 (2003) 2646-2652. |
| [6] | M. Tomizawa, J.E. Casida, Selective toxicity of neonicotinoids attributable to specificity of insect and mammalian nicotinic receptors, Annu. Rev. Entomol. 48 (2003) 339-364. |
| [7] | A. Elbert, M. Schindler, R. Nauen, P. Jeschke, Overview of the status and global strategy for neonicotinoids, J. Agric. Food Chem. 59 (2011) 2897-2908. |
| [8] | S. Kagabu, Discovery of imidacloprid and further developments from strategic molecular designs, J. Agric. Food Chem. 59 (2011) 2887-2896. |
| [9] | M. Tomizawa, J.E. Casida, Molecular recognition of neonicotinoid insecticides: the determinants of life or death, Acc. Chem. Res. 42 (2009) 260-269. |
| [10] | A. Elbert, R. Nauen, Resistance of bemisia tabaci (Homoptera: Aleyrodidae) to insecticides in southern Spain with special referenceto neonicotinoids, Pest Manage. Sci. 56 (2000) 60-64. |
| [11] | K.D. Ninsin, Acetamiprid resistance and cross-resistance in the diamondback moth, plutella xylostella, Pest Manage. Sci. 60 (2004) 839-841. |
| [12] | D.M. Sanchez, R.M. Hollingworth, E.J. Grafius, D.D. Moyer, Resistance and crossresistance to neonicotinoid insecticides and spinosad in the Colorado potato beetle, leptinotarsa decemlineata (Say) (Coleoptera: Chrysomelidae), Pest Manage. Sci. 62 (2006) 30-37. |
| [13] | K.G. Gorman, G. Devine, J. Bennison, et al., Report of resistance to the neonicotinoid insecticide imidacloprid in trialeurodes vaporariorum (Hemiptera: Aleyrodidae), Pest Manage. Sci. 63 (2007) 555-558. |
| [14] | G. Arnold, J. Pistorius, T. Steeger, H. Thompson, Statement on the findings in recent studies investigating sub-lethal effects in bees of some neonicotinoids in consideration of the uses currently authorised in Europe, EFSA J. 10 (2012) 2752-2779. |
| [15] | Z. Ye, S. Xia, X. Shao, et al., Design, synthesis, crystal structure analysis, and insecticidal evaluation of phenylazoneonicotinoids, J. Agric. Food Chem. 59 (2011) 10615-10623. |
| [16] | W. Zhang, X. Yang, W. Chen, et al., Design, multicomponent synthesis, and bioactivities of novel neonicotinoid analogues with 1,4-dihydropyridine scaffold, J. Agric. Food Chem. 58 (2010) 2741-2745. |
| [17] | N.Y. Chen, L.P. Ren, M.M. Zou, et al., Design, synthesis and insecticidal activity of spiro heterocycle containing neonicotinoid analogs, Chin. Chem. Lett. 25 (2014) 197-200. |
| [18] | C. Sun, J. Zhu, H. Wang, et al., Chiral 1,5-disubstituted 1,3,5-hexahydrotriazine-2-N-nitroimine analogues as novel potent neonicotinoids: synthesis, insecticidal evaluation and molecular docking studies, Eur. J. Med. Chem. 46 (2011) 11-20. |
| [19] | Y. Nakagawa, K. Takahashi, H. Kishikawa, et al., Classical and three-dimensional QSAR for the inhibition of [3H] ponasterone A binding by diacylhydrazine-type ecdysone agonists to insect Sf-9 cells, Bioorg. Med. Chem. 13 (2005) 1333-1340. |
| [20] | C. Minakuchi, Mode of action of nonsteroidal ecdysone agonists, diacylhydrazine analogs, J. Pestic. Sci. 30 (2005) 228-238. |
| [21] | X. Liu, L. Zhang, J.G. Tan, H.H. Xu, Design and synthesis of N-alkyl-N'-substituted 2,4-dioxo-3,4-dihydropyrimidin-1-diacylhydrazine derivatives as ecdysone receptor agonist, Bioorg. Med. Chem. 21 (2013) 4687-4697. |
| [22] | Z. Huang, Y. Liu, Y. Li, et al., Synthesis, crystal structures, insecticidal activities, and structure-activity relationships of novel N'-tert-Butyl-N'-substituted-benzoyl-N-[di(octa) hydro] benzofuran{(2,3-dihydro) benzo[1,3] ([1,4]) dioxine} carbohydrazide derivatives, J. Agric. Food Chem. 59 (2011) 635-644. |
| [23] | W.S. Abbott, A method of computing the effectiveness of an insecticide, J. Econ. Entomol. 18 (1995) 265-267. |
| [24] | T.T. Talley, M. Harel, R.E. Hibbs, et al., Atomic interactions of neonicotinoid agonists with AChBP: molecular recognition of the distinctive electronegative pharmacophore, Proc. Natl. Acad. Sci. U. S. A. 105 (2008) 7606-7611. |
| [25] | H.X. Duan, W.W. Zhang, J. Zhao, et al., A novel halogen bond and a better-known hydrogen bond cooperation of neonicotinoid and insect nicotinic acetylcholine receptor recognition, J. Mol. Model. 18 (2012) 3867-3875. |
| [26] | L. Sun, Y. Ling, C. Wang, et al., Synthesis and biological activities of E-β-Farnesene analogues containing substituent nitroguanidine, Chin. J. Org. Chem. 31 (2011) 2061-2066. |
| [27] | J.D. Schmitt, C.G.V. Sharples, W.S. Caldwell, Molecular recognition in nicotinic acetylcholine receptors: the importance of π-cation interactions, J. Med. Chem. 42 (1999) 3066-3074. |





