b College of Chemistry and Chemical Engineering, Nantong University, Nantong 226019, China
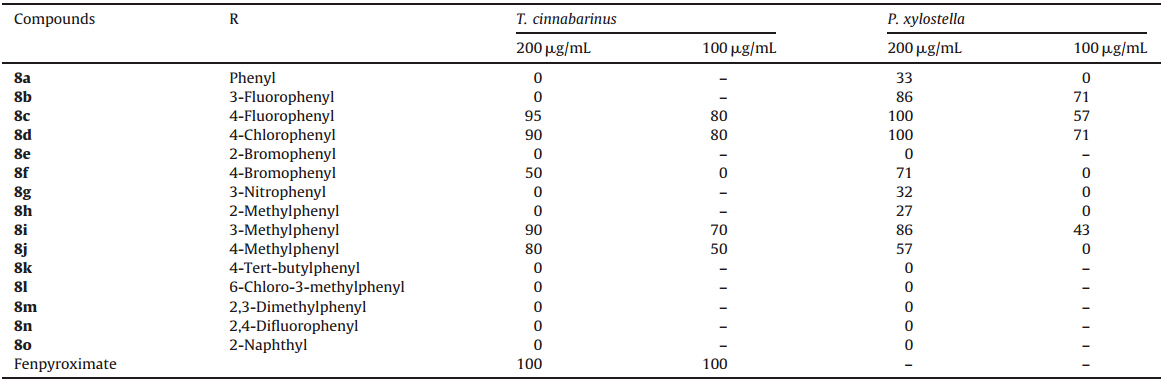
During the past two decades,different classes of pyrazole oxime compounds have been investigated and many of them are found to possess broad spectrum of bioactivities such as fungicidal [1, 2, 3], acaricidal [4],insecticidal [5],anti-TMV [6, 7],antibacterial [8],and anti-inflammatory activities [9]. For example,fenpyroximate (in Fig. 1),a well-known agricultural acaricide bearing a pyrazole oxime moiety in the structure,has been reported to exhibit strong acaricidal activity against several phytophagous mites on diverse crops [10]. As a result,pyrazole oxime containing heterocycles became a focus of chemical and pharmaceutical research.
Moreover,thiazole derivatives due to their unique chemical and structural properties have drawn much attention recently and are widely used in the fields of pesticides and medicines. Some thiazole compounds were also synthesized as potential insecticides [11, 12] and fungicides [13]. Some thiazole-containing derivatives also showed plant growth regulatory [14],herbicidal [15],antimicrobial [16],and anti-inflammatory [17] activities. Recently,Wanget al. [18] reported that some 2-cyanoacrylate compounds carrying a benzyloxy-linked thiazole unit displayed good bioactivities. This gave a great impetus to the search for biologically active molecules bearing substituted thiazole group.
Inspired by these facts,we envisioned that the introduction of the highly substituted thiazole moiety to the parent pyrazole oxime scaffold might afford some new compounds (Fig. 1) possessing wide spectrum bioactivities such as acaricidal and insecticidal activity. We report herein the synthesis and biological evaluation of a variety of novel pyrazole oximes carrying substituted thiazole unit.
|
Download:
|
| Fig. 1. Strategic design of the title compounds. | |
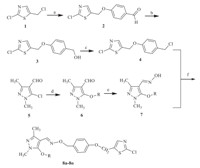
The synthetic route for the targeted compounds is shown in Scheme 1. Starting from 2-chloro-5-chloromethylthiazole 1, intermediates 2,3,4 were prepared in good yields according to the reported protocols [18]. Intermediate 5 was conveniently obtained by a similar method in the literature [19]. The intermediate aldehyde 6 was successively synthesizedvia the reaction of the corresponding aldehyde 5 with the respective substituted phenols or 2-naphthalenol [19, 20]. Compound 6 was further converted to the oxime 7 by a reaction with hydroxylamine hydrochloride using potassium hydroxide as a base. Finally, treatment of oxime 7 with the key intermediate 4 under basic conditions afforded the desired compounds 8a -8oin satisfactory yields. Optimized reaction conditions were adopted to synthesize a class of new pyrazole oxime derivatives containing substituted thiazole moiety. Their biological activity against Tetranychus cinnabarinusandPlutella xylostellawas evaluated according to the method described in literature [21, 22, 23]. 3. Results and discussion
All the final derivatives 8a-8o were purified by column chromatography on silica gel with the solvent system of ethyl acetate and petroleum ether (60-90°C),and their structures were fully identified by 1HNMR,13C NMR and elemental analysis. The details are given in Supporting information. The 1H NMR spectrumof 8a showed a singlet peak at δ2.37 attributed to the methyl protons at the 3-position of the pyrazole ring. A singlet at &delat;3.58 is due to the methyl protons at the 1-position of the pyrazole ring. Two singlets appeared in 5.14 and 4.93 ppm are due to the two methylene protons in the molecule. The presence of two singlet signals at δ7.51 and 7.78 are assignable to thiazole-4-CH and CH55N protons, respectively. In the 13C NMR spectrum of8a ,the peak belonging to CH55N group is observed at &delat;152.7. The biological activity data are listed in Table 1. Fenpyroximate was used as a positive control. As shown in Table 1,some designed compounds displayed promising acaricidal activity against T. cinnabarinus at a concentration of 200mg/mL. For example,compounds 8c, 8d,and 8i achieved around 90% inhibition againstT. cinnabarinus,which was comparable to that of the control Fenpyroximate. Additionally,compounds 8c, 8d, 8i and8jshowed moderate to good inhibition againstT. cinnabarinus when the dosage was reduced to 100mg/mL. Encouragingly,some of the designed compounds were also active againstP. xylostellaat the concentration of 200ug/mL. Among them,compounds 8b, 8c, 8d, and 8i displayed good to excellent insecticidal activity against P. xylostellawith inhibitory values of 86%,100%,100%,and 86%, respectively. Moreover,compounds8b, 8c,and 8d had moderate insecticidal activity againstP. xylostellaat the dosage of 100mg/mL. The data presented in Table 1 also showed that 4-fluoro substituted compound 8c and 4-chloro substituted analogue 8dwere more potent againstT. cinnabarinusandP. xylostella than other oxime derivatives. All the above data indicated that the biological activity spectrum of pyrazole oxime derivatives was significantly improved viathe introduction of the substituted thiazole moiety. These studies represent an important basis for the development of novel pesticides in future.
|
Download:
|
| Scheme 1. Reagents and conditions: (a)p-hydroxybenzaldehyde,potassium carbonate,ethanol,reflux for 8 h; (b) NaBH4(2.0 equiv.),ethanol,0°C for 1 h,r.t. for 2 h; (c) thionyl chloride (1.5 equiv.),dichloromethane,0°C for 30 min,r.t. for 12 h; (d) substituted phenols or 2-naphthalenol,potassium hydroxide,DMF,40°C for 2 h,110°C for 4-12 h; (e) hydroxylamine hydrochloride,potassium hydroxide,methanol,reflux for 6-12 h; (f) potassium carbonate,acetonitrile,reflux for 8-24h. | |
| Table 1 Acaricidal and insecticidal activities of compounds 8a -8o(mortality,%). |
In summary,we have achieved the synthesis of a series of new pyrazole oxime derivatives bearing substituted thiazole moiety. Preliminary bioassay demonstrated that some of the targeted compounds possessed insecticidal property besides acaricidal activity. Particularly,compounds 8c and 8ddisplayed relatively good acaricidal activity against T. cinnabarinus and potential insecticidal activity against P. xylostellaat the testing concentrations. Further structural modifications and bioactivity investigations on these new active compounds are currently in progress. Acknowledgments
This work was financial supported by the National Natural Science Foundation of China (No. 21202089),China Postdoctoral Science Foundation (No. 2013M531145),and the Research Foundation of the Six People Peak of Jiangsu Province (No. 2013-SWYY-013). Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.06.011.
| [1] | Y. Li, H.Q. Zhang, J. Liu, X.P. Yang, Z.J. Liu, Stereoselective synthesis and antifungal activities of (E)-α-(methoxyimino)benzeneacetate derivatives containing 1,3,5-substituted pyrazole ring, J. Agric. Food Chem. 54 (2006) 3636-3640. |
| [2] | P.L. Zhao, F. Wang, M.Z. Zhang, et al., Synthesis, fungicidal, and insecticidal activities of β-methoxyacrylate-containing N-acetyl pyrazoline derivatives, J. Agric. Food Chem. 56 (2008) 10767-10773. |
| [3] | P.L. Zhao, L. Wang, X.L. Zhu, et al., Subnanomolar inhibitor of cytochrome bc1 complex designed by optimizing interaction with conformationally flexible residues, J. Am. Chem. Soc. 132 (2010) 185-194. |
| [4] | H.J. Song, Y.X. Liu, L.X. Xiong, et al., Design, synthesis, and insecticidal evaluation of newpyrazole derivatives containing imine, oxime ether, and dihydroisoxazoline groups based on the inhibitor binding pocket of respiratory complex I, J. Agric. Food Chem. 61 (2013) 8730-8736. |
| [5] | X.M. Zou, M. Liu, H.Z. Yang, et al., Preparation of pyridine oxime ether derivatives as pesticides and acaricides, CN 101659656, 2010. |
| [6] | G.P. Ouyang, X.J. Cai, Z. Chen, et al., Synthesis and antiviral activities of pyrazole derivatives containing an oxime moiety, J. Agric. Food Chem. 56 (2008) 10160-10167. |
| [7] | G.P. Ouyang, Z. Chen, X.J. Cai, et al., Synthesis and antiviral activity of novel pyrazole derivatives containing oxime esters group, Bioorg. Med. Chem. 16 (2008) 9699-9707. |
| [8] | L.S. Bai, Y. Wang, X.H. Liu, H.L. Zhu, B.A. Song, Novel dihydropyrazole derivatives linked with multi(hetero)aromatic ring: synthesis and antibacterial activity, Chin. Chem. Lett. 20 (2009) 427-430. |
| [9] | R.R. Ranatunge, M. Augustyniak, U.K. Bandarage, et al., Synthesis and selective cyclooxygenase-2 inhibitory activity of a series of novel, nitric oxide donorcontaining pyrazoles, J. Med. Chem. 47 (2004) 2180-2193. |
| [10] | K. Motoba, H. Nishizawa, T. Suzuki, et al., Species-specific detoxification metabolism of fenpyroximate, a potent acaricide, Pestic. Biochem. Physiol. 67 (2000) 73-84. |
| [11] | P. Maienfisch, H. Huerlimann, A. Rindlisbacher, et al., The discovery of thiamethoxam: a second-generation neonicotinoid, Pest Manag. Sci. 57 (2001) 165-176. |
| [12] | H. Uneme, K. Iwanaga, N. Higuchi, et al., Synthesis and insecticidal activity of nitroguanidine derivatives, Pestic. Sci. 55 (1999) 202-205. |
| [13] | D.Y. Hu, B.A. Song, W. He, Progresses in the synthesis and biological activity of thiazole derivatives, Chin. J. Synth. Chem. 14 (2006) 319-328. |
| [14] | X. Qin, H.B. Yu, H. Dai, et al., Synthesis and plant-growth regulatory activities of novel imine derivatives containing 1H-1,2,4-triazole and thiazole rings, Chin. Chem. Lett. 21 (2010) 283-286. |
| [15] | L.L. Jiang, Y. Tan, X.L. Zhu, et al., Design, synthesis, and 3D-QSAR analysis of novel 1,3,4-oxadiazol-2(3H)-ones as protoporphyrinogen oxidase inhibitors, J. Agric. Food Chem. 58 (2010) 2643-2651. |
| [16] | H.M. Refat, A.A. Fadda, Synthesis and antimicrobial activity of some novel hydrazide, benzochromenone, dihydropyridine, pyrrole, thiazole and thiophene derivatives, Eur. J. Med. Chem. 70 (2013) 419-426. |
| [17] | A. Zablotskaya, I. Segal, A. Geronikaki, et al., Synthesis, physicochemical characterization, cytotoxicity, antimicrobial, anti-inflammatory and psychotropic activity of new N-[1,3-(benzo)thiazol-2-yl]-w-[3,4-dihydroisoquinolin-2(1H)-yl]alkanamides, Eur. J. Med. Chem. 70 (2013) 846-856. |
| [18] | T.T. Wang, G.F. Bing, X. Zhang, et al., Synthesis and herbicidal activities of 2-cyano-3-benzylaminoacrylates containing thiazole moiety, Bioorg. Med. Chem. Lett. 20 (2010) 3348-3351. |
| [19] | H.J. Park, K. Lee, S.J. Park, et al., Identification of antitumor activity of pyrazole oxime ethers, Bioorg. Med. Chem. Lett. 15 (2005) 3307-3312. |
| [20] | T. Hideo, H. Hiroshi, N. Akira, et al., Pyrazole derivative and production thereof, Japan Patent 62053970, 1987. |
| [21] | Y. Zhao, C.H. Mao, Y.Q. Li, et al., Synthesis, crystal structure, and insecticidal activity of novel N-alkyloxyoxalyl derivatives of 2-arylpyrrole, J. Agric. Food Chem. 56 (2008) 7326-7332. |
| [22] | Q.Q. Zhao, Y.Q. Li, L.X. Xiong, et al., Design, synthesis and insecticidal activity of novel phenylpyrazoles containing a 2,2,2-trichloro-1-alkoxyethyl moiety, J. Agric. Food Chem. 58 (2010) 4992-4998. |
| [23] | A.H. Sayyed, J. Ferre, D.J. Wright, Mode of inheritance and stability of resistance to Bacillus thuringiensis var kurstaki in a diamondback moth (Plutella xylostella) population from Malaysia, Pest Manag. Sci. 56 (2000) 743-748. |