b State Key Laboratory of Natural and Biomimetic Drugs, Peking University, Beijing 100191, China
D-Amino acids (DAAs) are ubiquitous in various biological samples,which include foods,plants,prokaryotes,mammals and so on [1, 2, 3, 4]. They have important physiological functions and play important roles in several biological processes [5, 6, 7]. However, abnormal concentration of DAAs is harmful and causes psychiatric diseases,such as schizophrenia,aging and related neurodegeneration diseases [6]. Therefore,it is necessary to detect the concentration of DAAs in biological samples with great precision and accuracy. Various analytical techniques for the determination of DAAs have been reported,which included high performance liquid chromatography (HPLC) [8, 9, 10],gas chromatography (GC) [11, 12, 13],electrochemical detection [14, 15, 16, 17]. Recently,Kano and co-workers [14] reported a sensitive D-amino acid oxidase (DAAO)/ peroxidase (POD) bienzyme biosensor,and the low detection limit of D-alanine (D-Ala) is 2.0 × 10-6 mol L-1. Schmitt-Kopplin et al. [9] reported a method using ultra-high-performance liquid chromatography/ mass spectrometry (UPLC-MS) that could provide limits of detection at pmol L-1 level. Although these methods are sensitive,most of them need expensive and sophisticated instruments and complex procedures,which make them inconvenient and time-consuming.
In recent years,colorimetric sensors based on functionalized gold nanoparticles (AuNPs) have attracted increasing interest for their convenience of visual observation and simple operation [18, 19, 20, 21, 22, 23]. AuNPs exhibit extremely high extinction coefficients (108-10110 moL-1 L cm-1) [24, 30],strongly distance-dependent optical properties,and colors arising from Au-NPs at nmol L-1 concentrations allow them to be easily monitored by naked eyes without the aid of any advanced instruments. The localized surface plasmon resonance (LSPR) absorption of AuNPs in the visible wavelength range depends on their size,shape,surrounding medium and interparticle distances. Because the aggregated AuNPs are royal purple whilst the dispersed ones are ruby red, this distinct change in color provides a basis for the naked-eye detection methods. These visual methods with a high sensitivity have been employed for the detection of glucose [25],DNA [26], metal ions [27, 28, 29, 30],b-agonists [31, 32],antibiotics [33],melamine [34, 35, 36] and so on. However,up to now there are few reports on the colorimetric detection of DAAs using AuNPs. Development of the colorimetric detection of D-amino acids by AuNPs is still a great challenge.
Our group has reported a colorimetric method for the detection of Cu2+ based on the aggregation of silver or gold nanoparticles in the presence of 4-MBA [37]. It was shown that the presence of 4-MBA and Cu2+ as aggregation agents could induce the aggregation of AuNPs [37]. The -SH group could be easily modified onto the surface of AuNPs [28]. In previous studies,most methods were based on AuNPs being induced to aggregate by crosslinking agents in the presence of target analytes,resulting in a color change from ruby red to royal purple [30, 31, 32, 33]. However,DAAO could oxidize DAAs to generate H2O2 [14, 15, 16]. In the presence of H2O2,the -SH group of 4-MBA can be oxidized to form a disulfide (-S-S-) bond [38, 39, 40, 41]. Based on these facts,in this paper,the DAAO mediated colorimetric detection of DAAs was developed by interrupting the aggregation of AuNPs induced by 4-MBA and Cu2+ as aggregation agents. According to the color changing from royal purple to ruby red,the concentrations of DAAs could be easily observed by a naked eye without the aid of the expensive and sophisticated instruments,which is much simpler and more cost-effective than the other existing detection methods of DAAs as mentioned before. According to our knowledge,this is the first report on the colorimetric detection of DAAs by anti-aggregating AuNPs. 2. Experimental 2.1. Chemicals and apparatus
DAAO from porcine kidney was obtained from Sigma Aldrich (USA). Hydrogen tetrachloroaurate hydrate (HAuCl4·4H2O) was purchased from Sinopharm Chemical Reagent Co.,Ltd. (Shanghai, China). D-Ala,L-Ala andotheramino acids,vitaminCandglucosewere obtained from J&K Chemical Ltd. (Beijing,China). Amyloid β 1-42 (Aβ1-42) is synthesized by solid phase peptide synthesizer in SBS Genetech Co.,Ltd. (Beijing,China). The amino acid sequences of Aβ1-42 were DAEFRHDSGYEVHHQKLVFFAEDVGSNKGAIIGLMVGGVVIA. Trisodium citrate,NaOH and HCl were bought from Beijing Chemical Reagent Company (Beijing,China). The acetate buffer was 0.01mol L-1 sodiumacetate (pH 3.0-11.0),whichwas adjustedwith acetic acid or sodium hydroxide. All chemicals were of analytical grade and used as received. Working solutions were obtained by serially diluting the stock solution immediately prior to use. All chemicals used are prepared using highly pure water with a resistance of 18MΩcm.
The UV-vis spectra were acquired on a UV-2550 spectrophotometer (Shimadzu,Japan),using 1 cmpath length quartz cuvettes for measurements. The pH value of the solution was measured with a PB-10 pH meter (Sartorius,Germany). IR spectra were measured using a VERTEX 70 FT-IR Spectrometer (Bruker, Germany). Transmission electron microscopy (TEM) measurements were performed on an H-7500 (Hitachi,Japan) at 80 kV. 2.2. Probe preparation
The negatively charged AuNPs of about 13 nm in diameter were prepared by reducing HAuCl4 with sodium citrate according to a method in a previous report [31, 42, 43]. Briefly,15 μL of 38.8 × 10-3 mol L-1 trisodium citrate was added rapidly into a boiling solution of 150 μL 1.0 × 10-3 mol L-1 HAuCl4 under vigorously magnetic stirring. And the mixed solution was continually boiled under stirring for another 30 min to produce a ruby red colored solution. The solution was cooled to room temperature while being stirred continuously. Then the citratestabilized AuNPs solution was formed. The concentration of the AuNPs solution as determined by UV-vis spectrometry was 10 nmol L-1 [30]. The stock solution of AuNPs was stored in a refrigerator at 4°C. 2.3. Detection of DAA
This DAAO mediated colorimetric detection of D-Ala as a representative DAA was performed at 37 °C. 50 μL of 1 mg μL-1 DAAO,50 μL of D-Ala with different concentrations and 100 μL of 6.5 × 10-5 mol L-1 4-MBA were added into a 400 μL acetate buffer sequentially at 37 °C and the mixture was allowed to stir for 30 min. Then 50 μL of 0.01 mol L-1 CuCl2 and 600 μL of AuNPs were added to this solution under stirring and the mixture was allowed to stir for about 15 min to ensure the self-assembly of the 4-MBA onto the surface of AuNPs. The absorption spectra of the resulting solutions were measured with UV-vis spectrophotometry, and the color of the final solutions was recorded by photographs. L-Amino acids,vitamin C and glucose were chosen to investigate their interference in D-Ala detection,and the concentration of each interference analyte was 3.0 × 10-5 mol L-1. 2.4. Real samples preparation
The present of D-Ala in the proteins from the brain tissues of Alzheimer patients has been reported [44]. And the selection of DAA peptides that bind to Aβ1-42 has attracts a lot of attention [45]. Based on this,Aβ1-42 was synthesized by solid phase peptide synthesizer as the real samples. And the sample was directly spiked with certain amounts of D-Ala standard solution. The preparation of these real samples was similar to those reported in previous literature [46]. Firstly,100 μL of 6 mol L-1 HCl and 100 μL of 1.0 × 10-4 mol L-1 Aβ1-42 samples were added to the digestion tubes,which were filled with nitrogen gas,then vibrating for 1 min. Secondly,the tubes were placed in a drying oven at 60 °C for 22 h to hydrolyze the samples. After hydrolysis,the samples were carefully evaporated by nitrogen blowing. Lastly,residues were dissolved in 1.0 μL of pH 7.0 acetate buffers for analysis. 3. Results and discussion 3.1. The mechanism of the sensing system
In this study,the principle of DAA colorimetric sensor is illustrated in Fig. 1. Because of the electrostatic repulsion between the negatively charged capping agents of sodium citrate,the negatively charged AuNPs are stable in the aqueous solution even with a high concentration of CuCl2. Fig. 1 shows that 4-MBA will be easily modified onto the surface of these AuNPs through the Au-S covalent bond if no DAA is present [28, 37]. The presence of Cu2+ and 4-MBA could induce the aggregation of AuNPs via electrostatic interactions between the 4-MBA and Cu2+,resulting in a color change from ruby red to royal purple [37]. However,DAAO could oxidize DAAs to generate H2O2 [14, 15, 16]. In the presence of H2O2,the -SH group of 4-MBA can be oxidized to form a disulfide (-S-S-) bond [38, 39, 40, 41]. Based on these facts,interestingly,if 4-MBA, which can be used as an aggregation agent with Cu2+ to induce an observable aggregation of AuNPs,is oxidized by H2O2 from the oxidization of a DAA by DAAO,the color of the AuNPs solution will be recovered. As the DAA concentration increases,additional H2O2 could change the color of AuNPs from royal purple to the original ruby red. The color change is parallel to the change of AuNPs from the aggregation to the dispersion state. This DAAO mediated colorimetric detection of DAAs,which was developed by interrupting the aggregation of AuNPs,can be applicable to measure other DAAs. The detection limit of D-Ala was as low as 7.5 × 10-8 mol L-1,which is lower than another DAAO mediated detection method [14, 15, 16]. In addition,it has been successfully applied to determine the concentration of D-Ala in real Aβ1-42 samples.

|
Download:
|
| Fig. 1.Schematic illustration of the anti-aggregation of AuNPs induced by DAA. | |
The FT-IR spectra of pure 4-MBA and 4-MBA modified AuNPs (4-MBA-AuNPs) are shown in Fig. 2. Comparing these FT-IR spectra,the characteristic absorption peak of -SH at 2524 cm-1 in pure 4-MBA had disappeared in the FT-IR spectra of 4-MBA-AuNPs. This indicated that 4-MBA had been successfully modified onto the surface of AuNPs via the -SH group in 4-MBA,which was similar to the observation from a previous report [37].

|
Download:
|
| Fig. 2.FT-IR spectra of (a) 4-MBA–AuNPs and (b) pure 4-MBA. | |
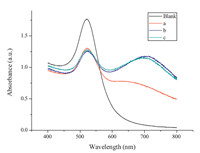
The 4-MBA as a dressing agent can induce the aggregation of AuNPs via electrostatic interactions between the 4-MBA and Cu2+ and causes the visible color changes of AuNPs. So,there should be an appropriate concentration of 4-MBA in the final colorimetric detection system. Based on the mechanism of the sensing system, the concentrations of 4-MBA should be as low as possible to induce the aggregation of AuNPs. Fig. 3 shows that the UV-vis absorption spectra of the AuNPs aggregation in the presence of different concentrations of 4-MBA. When the concentration of 4-MBA increased to over 5.2 × 10-6 mol L-1,the UV-vis spectrum of AuNPs could not be further changed,which means that the complete aggregation of AuNPs was formed. So,5.2 × 10-6 mol L-1 4-MBA was chosen for the following experiments.
|
Download:
|
| Fig. 3.UV–vis absorption spectra of the AuNPs aggregation in the presence of different concentrations of 4-MBA (blank: 0; (a) 4.2 × 10-6; (b) 5.2 × 10-6; (c) 6.2 × 10-6 mol L-1). | |
Ionic strength of the solution exerts a strong effect on the aggregation of sodium citrate coated negatively charged AuNPs. Fig. 4 shows that in the absence of 4-MBA,the absorbance ratio (A700 nm/A520 nm) of AuNPs was slightly enhanced with the increase of CuCl2 concentration. It might be attributed to the free sodium citrate in the solution restraining the aggregation of AuNPs induced by CuCl2. However,in the presence of 5.2 × 10-6 mol L-1 4-MBA,the absorbance ratio (A700 nm/A520 nm) of 4-MBA modified AuNPs sharply increased with increased concentration of CuCl2 but plateaued at 4 × 10-4 mol L-1. This suggests the presence of 4- MBA and Cu2+ induces the aggregation of AuNPs. Hence,the following experiments were conducted using 4 × 10-4 mol L-1 CuCl2.

|
Download:
|
| Fig. 4.Effect of CuCl2 on the absorbance ratio (A700 nm/A520 nm) of AuNPs (a) in the presence and (b) absence of 5.2 × 10-6 mol L-1 4-MBA under different concentrations of CuCl2: 0, 0.1, 0.2, 0.3, 0.4, 0.5, 0.8, and 1.0 mmol L-1, respectively. | |
The pH value for the colorimetric detection of DAA was optimized. Our results showed that this DAAO mediated colorimetric sensor could exhibit a maximum selectivity for DAA at pH 7.0,and these results are similar to those reported in literature [14, 16]. Therefore pH 7.0 was selected for further experiments. 3.6. Colorimetric detection of D-Ala
According to the previous reports [14, 15, 16],D-Ala was chosen as a representative DAA to be detected in this paper. To further demonstrate the assay for the DAAO mediated colorimetric visualization of DAA,the AuNPs solution was added to a mixture containing 4-MBA,DAAO and different amounts of D-Ala. Fig. 5 shows that the presence of D-Ala led to blue-shift of the peak at 700 nm and a new peak appeared at about 520 nm by UV-vis spectroscopy. The increase of D-Ala concentration could induce a decrease of maximal absorption of AuNPs at 700 nm,accompanying an obvious enhancement of the new peak at 520 nm,which indicated that the anti-aggregation of AuNPs has a relationship with the concentration of D-Ala. Under the optimum conditions,as the D-Ala concentration increases,a clear color change progression from royal purple to ruby red was observed (Fig. 5). Thereby,we can probably discriminate the concentration of D-Ala with a naked eye compared with the standard colorimetric picture.

|
Download:
|
| Fig. 5.UV–vis spectra and photographs of AuNPs with different concentration of DAla (a) 5.0 ×10-5; (b) 1.0 ×10-5; (c) 5.0 ×10-6; (d) 1.0 ×10-6; (e) 5.0 ×10-7; (f) 1.0 ×10-7 mol L-1; (g) 0. | |
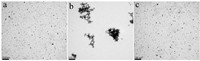
The direct evidence for D-Ala induced anti-aggregation of the AuNPs could be further supported by transmission electron microscopy (TEM) measurements. TEM measurement further confirmed that 4-MBA induced aggregation and the D-Ala induced anti-aggregation of AuNPs. Fig. 6 shows the TEM images of AuNPs in the presence and absence of 3.0 × 10-5 mol L-1 D-Ala. In the absence of D-Ala,4-MBA and Cu2+ could induce the aggregation of AuNPs in aqueous solution (Fig. 6 b). On the other hand,when D-Ala was present,AuNPs were dispersed (Fig. 6c). These results clearly suggested that DAAO could effectively oxidize D-Ala to generate H2O2,which can oxidize 4-MBA to form a -S-S- bond,leaving little or no chance for 4-MBA to bind on the surface of AuNPs. Thus,the aggregation role of 4-MBA was deactivated by D-Ala. Therefore, these results clearly indicate that the addition of trace DAA could readily lead to the anti-aggregation of AuNPs.
|
Download:
|
| Fig. 6.TEM of the original AuNPs in absence of D-Ala (a) and the AuNPs containing 5.2 ×10-6 mol L-1 4-MBA in the presence of 0 mol L-1 (b) and 3.0 ×10-6 mol L-1 D-Ala (c). | |
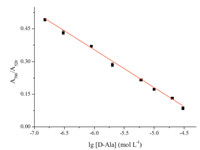
A quantitative analysis was performed by adding different concentrations of D-Ala into AuNPs and monitoring the max absorption by UV-vis spectroscopy. Fig. 7 shows that a linear correlation existed between the absorbance ratio (A700 nm/A520 nm) and base-10 logarithm of D-Ala concentration ranging from 1.5 × 10-7 mol L-1 to 3.0 × 10-5 mol L-1 (lg[D-Ala]) with a coefficient of 0.996. The detection limit was 7.5 × 10-8 mol L-1 (S/N = 3). This assay has distinctive advantages such as being much simpler and more cost-effective than the other existing detection methods of DAAs as mentioned before,and to the best of our knowledge,the detection limit is the lowest one among those have been reported so far in DAAO-based assays for DAA [8, 14, 15, 16, 17]. It suggests that this proposed method exhibited a higher sensitivity with a lower detection limit.
|
Download:
|
| Fig. 7.SA plot of the absorbance A700 nm/A520 nm of AuNPs versus the base-10 logarithm of D-Ala concentration: 1.5 ×10-7, 3×10-7, 9×10-7, 2×10-6, 6 ×10-6, 1 ×10-5, 2 ×10-5 and 3 ×10-5 mol L-1. | |
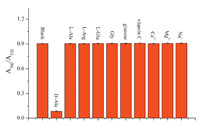
In additional experiments,some common potentially interfering substances in biological samples were investigated to test the selectivity of this method for colorimetric detection of DAA. The interferents with the same concentration of 3.0 × 10-5 mol L-1 were added into the solution of AuNPs. As shown in Fig. 8,no obvious change was obtained in the presence of vitamin C,glucose, Ca2+,Mg2+,Na+ ,glycine (Gly),L-glutamic acid (L-Glu),L-alanine (L-Ala) and L-arginine (L-Arg). The results demonstrated that this colorimetric sensors system is specific to DAA. This result was probably due to the fact that DAAOs have a high selectivity catalyzing the oxidative deamination of DAA to yield H2O2,and they are almost inactive toward the corresponding L-amino acid [47]. This mechanism of quantification of the levels of DAA using DAAO is similar with that reported in the literature [14, 15, 16]. It is worth noting that the DAAO in our visual detection method was from pig kidney. According to the previous report,pig kidney DAAO has specific catalytic activity to the neutral D-amino acids while it is inactive toward acidic D-amino acids [47]. Therefore,all the DAA detection methods mediated by pig kidney DAAO,including our visual detection method,cannot be used to detect acidic D-amino acids [14, 15, 16].
|
Download:
|
| Fig. 8.Absorption ratio A700 nm/A520 nm of AuNPs in the presence of D-Ala or other interferences (the concentrations of D-Ala and other interferences were 3.0 ×10-5 mol L-1). | |
The presence of D-Ala in the proteins in the brain tissue from Alzheimer patients has been reported [44]. And the selection of DAA peptides that bind to Aβ1-42 has attracted much attention [45]. Based on this,the Aβ1-42 was synthesized using a solid phase peptide synthesizer as the real samples. To assess the applicability of this colorimetric sensor for the analysis of real samples,a standard solution of D-Ala was added into an Aβ1-42 solution,and then detected utilizing DAAO mediated colorimetric method. The results were shown in Table 1,it suggests that this colorimetric detection of DAA is a practical tool for the determination of DAA in real biological samples.
| Table 1 The recovery of D-Ala under different concentrations in Ab1–42real samples. |
In summary,based on the anti-aggregation of AuNPs,a novel colorimetric sensor for the highly sensitive and selective detection of DAA was proposed. DAAO could oxidize DAAs to generate H2O2,which could further oxidize the -SH group in 4-MBA to form an -S-S- bond. So the aggregation role of 4-MBA was deactivated by DAA,resulting in a color change from royal purple to ruby red. Therefore,the concentration of the DAA could be determined by a UV-vis spectrometry or naked eyes. Our data show that a sensitive detection limit of D-Ala is as low as 7.5 × 10-8 mol L-1. The absorbance ratio (A700 nm/A520 nm) is linear with the base-10 logarithm of the concentration of D-Ala ranging from 1.5 × 10-7 mol L-1 to 3.0 × 10-5 mol L-1 with a coefficient of 0.996. In addition,the coexisting substances including other L-amine acid and metal ions did not affect the determination of DAA. The proposed method could be successfully applied to the determination of D-Ala in real Aβ1-42 samples with a high recovery rate of 98.5%. Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 21272263),the State Key Laboratory of Natural and Biomimetic Drugs (No. K20130206),the University of Chinese Academy of Sciences (No. 08JT011J01) and the Twelfth Five-Year Plan for National Sciences &Technology Support Project (No. 2012BAI37B03)
| [1] | M. Friedman, Chemistry, nutrition, and microbiology of D-amine acids, J. Agric. Food Chem. 47 (1999) 3457-3479. |
| [2] | C. Henneberger, T. Papouin, S.H.R. Oliet, et al., Long-term potentiation depends on release of D-serine from astrocytes, Nature 463 (2010) 232-236. |
| [3] | I. Azua, I. Goiriena, Z. Bana, et al., Release and consumption of D-amino acids during growth of marine prokaryotes, Microb. Ecol. 67 (2014) 1-12. |
| [4] | V. Vranova, H. Zahradnickova, D. Janous, et al., The significance of D-amino acids in soil, fate and utilization by microbes and plants: review and identification of knowledge gaps, Plant Soil 354 (2012) 21-39. |
| [5] | S.A. Fuchs, R. Berger, L.W.J. Klomp, et al., D-amino acids in the central nervous system in health and disease, Mol. Genet. Metab. 85 (2005) 168-180. |
| [6] | H. Wolosker, E. Dumin, L. Balan, V.N. Foltyn, D-amino acids in the brain: D-serine in neurotransmission and neurodegeneration, FEBS J. 275 (2008) 3514-3526. |
| [7] | G.H. Fisher, L. Petrucelli, C. Gardner, et al., Free D-amino acids in human cerebrospinalfluid of Alzheimer-disease, multiple-sclerosis, and healthy control subjects, Mol. Chem. Neuropathol. 23 (1994) 115-124. |
| [8] | S. Kato, Y. Kito, H. Hemmi, T. Yoshimura, Simultaneous determination of D-amino acids by the coupling method of D-amino acid oxidase with high-performance liquid chromatography, J. Chromatogr. B 879 (2011) 3190-3195. |
| [9] | C. Mueller, J.R. Fonseca, T.M. Rock, S. Krauss-Etschmann, P. Schmitt-Kopplin, Enantioseparation and selective detection of D-amino acids by ultra-high-performance liquid chromatography/mass spectrometry in analysis of complex biological samples, J. Chromatogr. A 1324 (2014) 109-114. |
| [10] | Y. Gogami, K. Okada, T. Oikawa, High-performance liquid chromatography analysis of naturally occurring D-amino acids in sake, J. Chromatogr. B 879 (2011) 3259-3267. |
| [11] | H. Brückner, A. Schieber, Determination of free D-amino acids in mammalia by chiral gas chromatography-mass spectrometry, J. High Resolut. Chromatogr. 23 (2000) 576-582. |
| [12] | R. Patzold, H. Bruckner, Gas chromatographic determination and mechanism of formation of D-amino acids occurring in fermented and roasted cocoa beans, cocoa powder, chocolate and cocoa shell, Amino Acids 31 (2006) 63-72. |
| [13] | R. Patzold, A. Schieber, H. Bruckner, Gas-chromatographic quantification of free Damino acids in higher vertebrates, Biomed. Chromatogr. 19 (2005) 466-473. |
| [14] | C.H. Nieh, Y. Kitazumi, O. Shirai, K. Kano, Sensitive D-amino acid biosensor based on oxidase/peroxidase system mediated by pentacyanoferrate-bound polymer, Biosens. Bioelectron. 47 (2013) 350-355. |
| [15] | S. Lata, B. Batra, C.S. Pundir, Construction of D-amino acid biosensor based on Damino acid oxidase immobilized onto poly (indole-5-carboxylic acid)/zinc sulfide nanoparticles hybrid film, Process Biochem. 47 (2012) 2131-2138. |
| [16] | S. Lata, B. Batra, P. Kumar, et al., Construction of an amperometric D-amino acid biosensor based on D-amino acid oxidase/carboxylated mutliwalled carbon nanotube/ copper nanoparticles/polyalinine modified gold electrode, Anal. Biochem. 437 (2013) 1-9. |
| [17] | E. Rosini, G. Molla, C. Rossetti, et al., A biosensor for all D-amino acids using evolved D-amino acid oxidase, J. Biotechnol. 135 (2008) 377-384. |
| [18] | Y.C. Cao, R.C. Jin, S. Thaxton, et al., A two-color-change, nanoparticle-based method for DNA detection, Talanta 67 (2005) 449-455. |
| [19] | H. Chi, B.H. Liu, G.J. Guan, et al., A simple, reliable and sensitive colorimetric visualization of melamine in milk by unmodified gold nanoparticles, Analyst 135 (2010) 1070-1075. |
| [20] | F. Li, Y. Feng, C. Zhao, et al., Simple colorimetric sensing of trace bleomycin using unmodified gold nanoparticles, Biosens. Bioelectron. 26 (2011) 4628-4631. |
| [21] | C.D. Medley, J.E. Smith, Z. Tang, et al., Gold nanoparticle-based colorimetric assay for the direct detection of cancerous cells, Anal. Chem. 80 (2008) 1067-1072. |
| [22] | H. Li, Y.W. Yang, Gold nanoparticles functionalized with supramolecular macrocycles, Chin. Chem. Lett. 24 (2013) 545-552. |
| [23] | B.H. Wu, H.Y. Yang, H.Q. Huang, et al., Solvent effect on the synthesis of monodisperse amine-capped Au nanoparticles, Chin. Chem. Lett. 24 (2013) 457-462. |
| [24] | S. Link, M.A. El-Sayed, Spectral properties and relaxation dynamics of surface plasmon electronic oscillations in gold and silver nanodots and nanorods, J. Phys. Chem. B 103 (1999) 8410-8426. |
| [25] | Y.P. Li, L. Jiang, T. Zhang, et al., Colorimetric detection of glucose using a boronic acid derivative receptor attached to unmodified AuNPs, Chin. Chem. Lett. 25 (2014) 77-79. |
| [26] | R. Elghanian, J.J. Storhoff, R.C. Mucic, et al., Selective colorimetric detection of polynucleotides based on the distance-dependent optical properties of gold nanoparticles, Science 277 (1997) 1078-1081. |
| [27] | C.Y. Lin, C.J. Yu, Y.H. Lin, W.L. Tseng, Colorimetric sensing of silver(I) and mercury(II) ions based on an assembly of Tween 20-stabilized gold nanoparticles, Anal. Chem. 82 (2010) 6830-6837. |
| [28] | N. Ding, H. Zhao, W.B. Peng, et al., A simple colorimetric sensor based on antiaggregation of gold nanoparticles for Hg2+ detection, Colloids Surf. A-Physicochem. Eng. Aspect 395 (2012) 161-167. |
| [29] | J.S. Lee, M.S. Han, C.A. Mirkin, Colorimetric detection of mercuric ion (Hg2+) in aqueous media using DNA-functionalized gold nanoparticles, Angew. Chem. Int. Ed. 46 (2007) 4093-4096. |
| [30] | Y. Xue, H. Zhao, Z.J. Wu, et al., Colorimetric detection of Cd2+ using gold nanoparticles cofunctionalized with 6-mercaptonicotinic acid and L-cysteine, Analyst 136 (2011) 3725-3730. |
| [31] | Y. Zhou, P.L. Wang, X.O. Su, et al., Colorimetric detection of ractopamine and salbutamol using gold nanoparticles functionalized with melamine as a probe, Talanta 112 (2013) 20-25. |
| [32] | X.F. Zhang, H. Zhao, Y. Xue, et al., Colorimetric sensing of clenbuterol using gold nanoparticles in the presence of melamine, Biosens. Bioelectron. 34 (2012) 112-117. |
| [33] | X.F. Zhang, Y. Zhang, H. Zhao, et al., Highly sensitive and selective colorimetric sensing of antibiotics in milk, Anal. Chim. Acta 778 (2013) 63-69. |
| [34] | X.F. Zhang, Z.J. Wu, Y. Xue, et al., Colorimetric detection of melamine based on the interruption of the synthesis of gold nanoparticles, Anal. Methods 5 (2013) 1930-1934. |
| [35] | Z.J. Wu, H. Zhao, Y. Xue, et al., Colorimetric detection of melamine during the formation of gold nanoparticles, Biosens. Bioelectron. 26 (2011) 2574-2578. |
| [36] | Q.A. Cao, H. Zhao, Y.J. He, et al., Hydrogen-bonding-induced colorimetric detection of melamine by nonaggregation-based Au-NPs as a probe, Biosens. Bioelectron. 25 (2010) 2680-2685. |
| [37] | Y. Zhou, H. Zhao, Y.J. He, N. Ding, Q. Cao, Colorimetric detection of Cu2+ using 4-mercaptobenzoic acid modified silver nanoparticles, Colloid Surf. A-Physicochem. Eng. Aspect 391 (2011) 179-183. |
| [38] | Y.C. Shiang, C.C. Huang, H.T. Chang, Gold nanodot-based luminescent sensor for the detection of hydrogen peroxide and glucose, Chem. Commun. (2009) 3437-3439. |
| [39] | A.R. Quesada, R.W. Byrnes, S.O. Krezoski, et al., Direct reaction of H2O2 with sulfhydryl groups in HL-60 cells: zinc-metallothionein and other sites, Arch. Biochem. Biophys. 334 (1996) 241-250. |
| [40] | B. Cardey,M. Enescu, Selenocysteine versus cysteine reactivity: a theoretical study of their oxidation by hydrogen peroxide, J. Phys. Chem. A 111 (2007) 673-678. |
| [41] | J. Wang, D.M. Wang, Y.F. Li, Study of cysteine modified gold nanoparticles as a colorimetric detection platform for oxidants, Chin. Sci. Bull. 56 (2011) 1196-1203. |
| [42] | L.F. Yuan, Y.J. He, Effect of surface charge of PDDA-protected gold nanoparticles on the specificity and efficiency of DNA polymerase chain reaction, Analyst 138 (2013) 539-545. |
| [43] | X.H. Ji, X.N. Song, J. Li, et al., Size control of gold nanocrystals in citrate reduction: the third role of citrate, J. Am. Chem. Soc. 129 (2007) 13939-13948. |
| [44] | A. Daniello, A. Vetere, G.H. Fisher, et al., Presence of D-alanine in proteins of normal and Alzheimer human brain, Brain Res. 592 (1992) 44-48. |
| [45] | K. Wiesehan, K. Buder, R.P. Linke, et al., Selection of D-amino-acid peptides that bind to Alzheimer's disease amyloid peptide Aβ1-42 by mirror image phage display, Chembiochem 4 (2003) 748-753. |
| [46] | Y.S. Shim, W.J. Yoon, J. Ha, et al., Method validation of 16 types of structural amino acids using an automated amino acid analyzer, Food Sci. Biotechnol. 22 (2013) 1567-1571. |
| [47] | S.V. Khoronenkova, V.I. Tishkov, D-amino acid oxidase: physiological role and applications, Biochemistry (Mosc.) 73 (2008) 1511-1518. |





