b Department of Pharmacy, The Second Hospital of Jilin University, Jilin 130041, China;
c China Resources Double-Crane Pharmaceutical Co., Ltd., Beijing 100121, China
The fibroblast growth factor receptor 1 (FGFR-1) is a transmembrane tyrosine kinase receptor (TKR) that belongs to the immunoglobulin (Ig) superfamily [1]. FGFR-1 was first cloned and identified by Leeet al.,as a member of the FGFR superfamily [2],which consists of four distinct isoforms (FGFR-1 to FGFR-4) found among several tissue types and expressed to different extents under varying conditions [3, 4]. The pivotal roles of the signaling of FGFRs in regulating key cellular processes of cell growth,proliferation,wound healing,embryogenesis and angiogenesis are well documented [3, 5, 6].
Of the 4 known human FGFRs,FGFR-1 is one of the most important members and has been deemed a strong candidate for drug development as an anti-tumor target [7, 8]. Since the 1990s,numerous small molecular inhibitors targeting the kinase domain of FGFR-1 have been reported [6],including pyridopyrimidines,indolinones,quinazolines/quinolines,indoles,and imidazopyridines. Despite the significant progress,very limited efforts have been devoted toward the development of FGFR-1 activators,especially small molecular activators,and none reported to date. In this paper,we report the identification of N2,N4-substitued-cycloalkyl[d]pyrimidine-2,4-diamines as first-generation small molecular activators of FGFR-1 kinase.
Inspired by the structure of pazopanib (1,Fig. 1) [9] as a representative anti-angiogenesis drug,recently we initiated a program aimed at seeking small molecular,multi-target,antiangiogenesis agents. In order to obtain novel analogs of pazopanib, two types of novel cycloalkyl[d]pyrimidine skeletons (2 and 3) derived fromthe pyrimidine linker of pazopanib,were designed,and a total of 16 analogs (2,3,Fig. 1) containing these two skeletons were synthesized,and tested in four pro-angiogenic kinase inhibitory assays (FGFR-1,VEGFR-2,PDGFR-&bate;,and c-KIT). Unexpectedly,our research identified 11 analogs that showed good kinase-selective activation effects on FGFR-1 to a certain degree. To the best of our knowledge,these activators are the first examples of a smallmolecular FGFR-1 activator,which might be used as an effective chemical probe for the study of FGFR-1 related signaling pathway.

|
Download:
|
| Fig. 1. Chemical structures of pazopanib (1) and cycloalkyl[d]pyrimidine analogs 2 and 3. | |
2.1. Chemistry
All purchased starting materials were used without further purification. Analytical thin-layer chromatography (TLC) was [HSGF 254 (150-200mm thickness; Yantai Huiyou Co.,China). Yields were not optimized. [Melting points were measured in capillary tubes on a SGW X-4 melting point apparatus without correction. Nuclear magnetic resonance (NMR) spectroscopy was performed on a Bruker AMX-400 NMR (IS as tetramethylsilane). Chemical shifts were reported in parts per million (ppm,δ) downfield from THS. Proton coupling patterns were described as singlet (s), doublet (d),triplet (t),quartet (q),multiplet (m),and broad (br). Low- and high-resolution mass spectra (LRMS and HRMS) were obtained with electric and electrospray ionization (EI and ESI) on a Finnigan MAT-95 and LCQ-DECA spectrometer. The purities of compounds 2 and 3was determined by Agilent 1100,and checked with a UV/vis detector setting λ= 254 nm.
The synthetic route of cycloalkyl[d]pyrimidine analogs 2 and 3 is described in Scheme 1. Various &bate;-ketoesters (5) and urea were placed in a domestic microwave oven and irradiated at a power of 450 W for 12 min to afford compounds 6,which were converted to the corresponding aryl chlorides (7) in the presence of POCl3. The 2-substituted-anilines (8) were nitrated to yield 2-substituted-5-nitroaniline (9). Cyclization of 9 with isoamyl nitrite in acetic acid to give 3-R1-6-nitroindazole (10),followed by methylation with dimethyl carbonate to give 2-methyl-3-R1-6-nitroindazole (11). Reduction of11 with tin (II) chloride gave 2-methyl-3-R1-6-aminoindazole (12),and condensation with 7 using basic conditions in THF and ethanol yielded key intermediate (13), followed by second methylation with dimethyl carbonate to give another key intermediate (14). Finally,a second condensation of 13 or 14 with various anilines was accomplished using acidic conditions in ethanol to yield the desired cycloalkyl[d]pyrimidines (2 and 3). Compound4was prepared by condensation of 14 with ethanol using acidic conditions.

|
Download:
|
| Scheme 1. Synthetic route of the target compounds 2 and 3. Reagents and conditions: (a) urea,MWI,450 w,12 min,yield 50%; (b) POCl3,r.t.,2 h,yield 46%; (c) conc.H2SO4, KNO3,0°C,1.5 h,yield 68%-72%; (d) isoamyl nitrite,AcOH,N2,r.t.,1.5 h,yield 88%-91%; (e) dimethyl carbonate,NaH,NMF,reflux,5 h,yield 28%-35%; (f) SnCl2,conc. H+Cl, DME,r.t.,3 h,yield 65%-76%; (g) NaHCO3,THF/EtOH (v/v = 1/4),reflux,50 h,yield 48%-60%; (h) R5-Ph-NH2,conc. H+Cl,EtOH,N2,reflux,24 h,yield 20%-46%; (i) conc. H+Cl, EtOH,N2,reflux,48 h,yield 23%. | |
5-[[4-[N-(2,3-Dimethyl-2H-indazol-6-yl)-N-methylamino]-6,7-dihydro-5H-cyclopenta[d]pyrimidin-2-yl]amino]-2-methylbenzenesulfonamide (2a): White solid,mp: 256-258°C,yield: 40% (last step),purity: 98% (tR 7.3 min,CH3OH/H2O = 80/20,v/v). 1H NMR (400 MHz,DMSO-d6): δ 10.39 (s,1H),8.36 (s,1H),7.80 (d,1H,J= 8.6 MHz),7.66 (d,1H,J= 8.5 MHz),7.53 (s,1H),7.46-7.36 (m,3H),7.00 (d,1H,J= 8.7 MHz),4.08 (s,3H),3.55 (s,3H),2.82-2.74 (m,2H),2.64 (s,3H),2.58 (s,3H),1.80-1.60 (m,4H). HRMS (EI)m/z calcd. for C24H27N7O2S(M+): 477.1947,found: 477.1948.
5-[[4-[N-(2-[Methyl-2H-indazol-6-yl)-N-methylamino]-6,7-dihydro-5H-cyclopenta[d]pyrimidin-2-yl]amino]-2-methyl-benzenesulfonamide (2b): White solid,mp: 262-264°C,yield: 46% (last step),purity: 99% (tR 6.1 min,CH3OH/H2O = 90/10,v/v). 1H NMR (400 MHz,DMSO-d6):δ9.35 (s,1H),8.65 (d,1H,J= 2.1 MHz), 8.37 (s,1H),7.73 (d,2H,J= 8.7 MHz),7.37 (s,1H),7.20 (m,3H),6.97 (dd,1H,J= 8.8,1.8 MHz),4.18 (s,3H),3.52 (s,3H),2.58 (t,2H, J= 7.5 MHz),1.79-1.74 (m,2H),1.66-1.57 (m,2H); HRMS (EI)m/z calcd. for C23H25N7O2S(M+): 463.179,found: 463.1788.
5-[[4-[(2,3-Dimethyl-2H-indazol-6-yl)amino]-6,7-dihydro-5Hcyclopenta[d]pyrimidin-2-yl]amino]-2-methyl-benzenesulfonamide (2c): Off-white solid,mp: 298-301°C,yield: 24% (last step), purity: 99% (tR 6.2 min,CH3OH/H2O = 90/10,v/v). 1H NMR (400 MHz,DMSO-d6): δ 10.40 (s,1H),9.94 (s,1H),7.86 (s,1H), 7.81 (s,1H),7.6 (d,1H,J= 6.2 MHz),7.68 (d,1H,J= 8.6 MHz),7.59 (s, 2H),7.21 (d,1H,J= 8.5 MHz),7.16 (d,1H,J= 6.3 MHz),4.05 (s,3H),3.55 (s,3H),2.95 (s,2H),2.91-2.78 (m,2H),2.61 (s,3H),2.56 (s,3H), 2.15 (s,2H); HRMS (EI)m/zcalcd. for C23H25N7O2S(M+): 463.179,found: 463.1789.
3-[[4-[N-(2,3-Dimethyl-2H-indazol-6-yl)-N-methylamino]-6,7-dihydro-5H-cyclopenta[d]pyrimidin-2-yl]amino]-benzenesulfonamide (2d): White solid,mp: 240-243°C,yield: 37% (last step), purity: 99% (tR 6.8 min,CH3OH/H2O = 80/20,v/v). 1H NMR (400 MHz,CDCl3): δ 8.25 (s,1H),7.72 (brs,1H),7.56 (d,1H, J= 8.6 MHz),7.42-7.34 (m,2H),7.20-7.07 (m,2H),6.91 (d,1H, J= 8.6 MHz),4.11 (s,3H),3.59 (s,3H),2.75-2.66 (m,2H),2.64 (s,3H), 1.91-1.84 (m,2H),1.76-1.64 (m,2H); HRMS (EI)m/zcalcd. for C23H25N7O2S(M+): 463.179,found: 463.1791.
3-[[4-[(2,3-Dimethyl-2H-indazol-6-yl)amino]-6,7-dihydro-5Hcyclopenta[d]pyrimidin-2-yl]amino]-benzenesulfonamide (2e): White solid,mp: 270-273°C,yield: 20% (last step),purity: 100% (tR 6.8 min,CH3OH/H2O = 80/20,v/v). 1H NMR (400 MHz,DMSOd6):d9.49 (drs,1H),8.06 (m,1H),7.57 (d,1H,J= 9.0 MHz),7.41 (d, 1H,J= 8.7 MHz),7.39 (s,2H),7.34 (s,1H),7.26 (dd,1H,J= 8.6, 2.5 MHz),7.22 (dd,1H,J= 9.1,2.5 MHz),4.00 (s,3H),2.8-2.77 (m,4H), 2.58 (s,3H),2.09-2.01 (m,2H); HRMS (EI) m/z calcd. for C22H23N7O2S(M+): 449.1634,found: 449.1632.
3-[[4-[N-(2,3-Dimethyl-2H-indazol-6-yl)-N-methylamino]-6,7-dihydro-5H-cyclopenta[d]pyrimidin-2-yl]amino]-4-methoxybenzenesulfonamide (2f): White solid,mp: 187-189°C,yield: 23% (last step),purity: 100% (tR 12.4 min,CH3OH/H2O = 80/20,v/v). 1H NMR (400 MHz,DMSO-d6):δ8.68 (s,1H),8.46 (s,1H),8.03 (s,1H), 7.69 (s,1H),7.56 (d,1H,J= 8.8 MHz),7.46 (d,1H,J= 8.7 MHz),7.43 (s, 2H),7.22-7.14 (m,2H),3.97 (s,3H),3.93 (s,3H),2.82-2.73 (m,4H), 2.56 (s,3H),2.08-2.00 (m,2H); HRMS (EI) m/z calcd. for C24H27N7O3S(M+): 493.1896,found: 493.1874.
N2-(3,4-Dimethoxyphenyl)-N4-(2,3-dimethyl-2H-indazol-6-yl)-N4-methyl-6,7-dihydro-5H-cyclopenta[d]pyrimidine-2,4-diamine (2g): Pink solid,mp: 210-214°C,yield: 20% (last step), purity: 100% (tR 12.1 min,CH3OH/H2O = 80/20,v/v). 1H NMR (400 MHz,CDCl3):δ7.59 (d,1H,J= 2.4H),7.56 (d,1H,J= 8.8 MHz), 7.37 (d,1H,J= 1.1 MHz),7.02 (dd,1H,J= 8.6 MHz,2.4 MHz),6.93 (1H,dd, J= 8.8,1.7 MHz),6.85 (d,1H,J= 8.7 MHz),4.13 (s,3H),3.93 (s,3H),3.89 (s,3H),3.59 (s,3H),2.73-2.62 (m,5H),1.90 (t,2H,J= 7.1 MHz),1.75- 1.66 (dd,2H,J= 14.6 MHz,7.5 MHz); HRMS (EI) m/z calcd. for C25H28N6O2(M+): 444.2274,found: 444.2276.
N2-(3-Bromohenyl)-N4-(2,3-dimethyl-2H-indazol-6-yl)-N4-methyl-6,7-dihydro-5H-cyclopenta[d]pyrimidine-2,4-diamine (2h): Pink solid,mp: 215-218°C,yield: 33% (last step),purity: 97% (tR 13.1 min,CH3OH/H2O = 80/20,v/v). 1H NMR (400 MHz,CDCl3): δ8.25 (s,1H),7.70 (brs,1H),7.56 (d,1H,J= 8.6 MHz),7.43-7.35 (m, 2H),7.19-7.07 (m,2H),6.91 (d,1H,J= 8.6 MHz),4.11 (s,3H),3.59 (s, 3H),2.75-2.66 (m,2H),2.64 (s,3H),1.90-1.85 (m,2H),1.76-1.64 (m,2H); HRMS (ESI)m/zcalcd. for C23H24BrN6[M+H]+ 463.1246, found: 463.12403.
N2-(3-Fluorohenyl)-N4-(2,3-dimethyl-2H-indazol-6-yl)-N4-methyl-6,7-dihydro-5H-cyclopenta[d]pyrimidine-2,4-diamine (2i): Pink solid,mp: 222-224°C,yield: 36% (last step),purity: 99% (tR 7.7 min,CH3OH/H2O = 80/20,v/v). 1H NMR (400 MHz,CDCl3): δ7.84 (d,1H,J= 12.1 MHz),7.55 (d,1H,J= 8.8 MHz),7.43 (brs,1H), 7.36 (d,1H,J= 1.7 MHz),7.24-7.12 (m,2H),6.91 (dd,1H,J=8.8, 1.8 MHz),6.71-6.62 (m,1H),4.11 (s,3H),3.58 (s,3H),2.69 (t,2H, J= 7.7 MHz),2.64 (s,3H),1.89 (t,2H,J= 7.2 MHz),1.71 (m,2H); HRMS (ESI) m/z calcd. for C23H24FN26[M+H]+: 403.2046,found: 403.2041.
5-[[4-[N-(2,3-Dimethyl-2H-indazol-6-yl)-N-methylamino]-5,6,7,8-tetrahydroquinazolin-2-yl]amino]-2-methyl-benzenesulfonamide (3a): White solid,mp: 316-320°C,yield: 27% (last step), purity: 97% (tR 11.1 min,CH3OH/H2O = 80/20,v/v). 1H NMR (400 MHz,DMSO-d6): δ10.30 (s,1H),8.38 (s,1H),7.80 (d,1H, J= 8.8 MHz),7.65 (d,1H,J= 8.2 MHz),7.46-7.38 (m,4H),7.03 (dd,1H, J= 8.7,1.6 MHz),4.07 (s,3H),3.54 (s,3H),2.70-2.64 (m,2H),2.64 (s, 3H),2.58 (s,3H),1.53 (s,4H),1.33 (s,2H); HRMS (EI)m/zcalcd. for C25H29N7O2S(M+): 491.2103,found: 491.2102.
5-[[4-[(2,3-Dimethyl-2H-indazol-6-yl)amino]-5,6,7,8-tetrahydroquinazolin-2-yl]amino]-2-methyl-benzenesulfonamide (3b): White solid,mp: 290-293°C,yield: 20% (last step),purity: 98% (tR 11.6 min,CH3OH/H2O = 80/20,v/v). 1H NMR (400 MHz,DMSOd6): δ 10.41 (s,1H),10.04 (s,1H),8.42 (s,1H),7.81 (d,1H, J= 8.7 MHz),7.65 (d,1H,J= 8.5 MHz),7.58 (s,1H),7.45-7.38 (m,3H), 7.02 (s,1H),4.10 (s,3H),2.86-2.75 (m,2H),2.65 (s,3H),2.58 (s, 3H),1.59-1.49 (m,4H),1.45-1.36 (m,2H); HRMS (EI)m/zcalcd. for C24H27N7O2S(M+): 477.1947,found: 477.1945.
3-[[4-[N-(2,3-Dimethyl-2H-indazol-6-yl)-N-methylamino]-5,6,7,8-tetrahydroquinazolin-2-yl]amino]-benzenesulfonamide (3c): Pale yellow solid,mp: 263-266°C,yield: 36% (last step), purity: 100% (tR 6.6 min,CH3OH/H2O = 90/10,v/v). 1H NMR (400 MHz,DMSO-d6): δ9.55 (s,1H),8.67 (s,1H),7.78 (d,1H, J= 7.8 MHz),7.65 (d,1H,J= 8.8 MHz),7.41 (t,1H,J= 7.9 MHz),7.32 (d, 1H,J= 7.8 MHz),7.23 (s,2H),7.01 (s,1H),6.85 (d,1H,J= 8.8 MHz),4.01 (s,3H),3.46 (s,3H),2.62 (t,2H,J= 6.0 MHz),2.59 (s,3H),1.68 (t,2H, J= 5.8 MHz),1.61-1.54 (m,2H),1.42-1.33 (m,2H); HRMS (EI)m/z calcd. for C24H27N7O2S(M+): 477.1947 (M+),found: 477.1945.
N2-(3,4-Dimethoxyphenyl)-N4-(2,3-dimethyl-2H-indazol-6-yl)-N4-methyl-5,6,7,8-tetrahydroquinazoline-2,4-diamine (3d): White solid,mp: 193-196°C,yield: 40% (last step),purity: 100% (tR 12.4 min,CH3OH/H2O = 80/20,v/v). 1H NMR (400 MHz,DMSOd6):δ8.96 (s,1H),7.68 (s,1H),7.64 (d,1H,J= 8.8 MHz),7.20 (d,1H, J= 8.6 MHz),6.99 (s,1H),6.88-6.80 (m,2H),4.01 (s,3H),3.72 (s,3H), 3.70 (s,3H),3.43 (s,3H),2.64-2.55 (m,5H),1.69 (s,2H),1.57 (s, 2H),1.38 (s,2H); HRMS (EI)m/z calcd. for C26H30N6O2 (M+): 458.243,found: 458.2426.
N2-(3-Bromophenyl)-N4-(2,3-dimethyl-2H-indazol-6-yl)-N4-methyl-5,6,7,8-tetrahydroquinazoline-2,4-diamine (3e): White solid,mp: 201-206°C,yield: 33% (last step),purity: 98% (tR 9.2 min,CH3OH/H2O = 90/10,v/v). 1H NMR (400 MHz,CDCl3): δ 8.23 (s,1H),7.51 (d,1H,J= 8.1 MHz),7.39 (d,1H,J= 8.0 MHz),7.27 (s, 1H),7.19 (s,1H),7.12 (d,1H,J= 8.9 MHz),7.07 (d,1H,J= 7.7 MHz),6.86 (d,1H,J= 9.1 MHz),4.08 (s,3H),3.54 (s,3H),2.71-2.64 (m,2H),2.60 (s,3H),1.79-1.72 (m,2H),1.68-1.58 (m,2H),1.49-1.35 (m,2H); HRMS (EI)m/z calcd. for C24H25BrN6(M+): 476.1324,found: 476.1316.
N2-(3-Fluorophenyl)-N4-(2,3-dimethyl-2H-indazol-6-yl)-N4-methyl-5,6,7,8-tetrahydroquinazoline-2,4-diamine (3f): White solid,mp: 169-172°C,yield: 25% (last step),purity: 99% (tR 12.2 min,CH3OH/H2O = 80/20,v/v). 1H NMR (400 MHz,CDCl3):δ 7.84 (d,1H,J= 12.2 MHz),7.55-7.47 (m,2H),7.23-7.14 (m,2H),6.86 (d,1H,J= 9.0 MHz),6.68 (t,2H,J= 8.0 MHz),4.09 (s,3H),3.55 (s,3H), 2.71-2.67 (m,2H),2.62 (s,3H),1.73 (s,2H),1.68 (s,2H),1.44-1.40 (m,2H); HRMS (EI)m/zcalcd. for C24H25FN6(M+): 416.2125,found: 416.2119.
N2-(3-Chloro-4-fluorophenyl)-N4-(2,3-dimethyl-2H-indazol-6-yl)-N4-methyl-5,6,7,8-tetrahydroquinazoline-2,4-diamine (3g): White solid,mp: 180-182°C,yield: 20% (last step),purity: 95% (tR 24.6 min,CH3OH/H2O = 95/5,v/v). 1H NMR (400 MHz,DMSOd6):d9.43 (s,1H),8.22 (dd,1H,J= 6.9,2.6 MHz),7.70-7.66 (m,1H), 7.65 (d,1H,J= 8.9 MHz),7.29 (t,1H,J= 9.1 MHz),7.01 (dd,1H,J= 9.2, 1.3 MHz),6.83 (dd,1H,J= 9.2,1.7 MHz),4.01 (s,3H),3.43 (s,3H),2.64- 2.59 (m,5H),2.59 (s,3H),1.72-1.61 (m,2H),1.61-1.50 (m,2H), 1.42-1.33 (m,2H); HRMS (ESI)m/zcalcd. for C24H24ClFN6[M+H]+]: 451.1813,found: 451.1815.
N-[Methyl-N-(2,3-dimethyl-2H-indazol-6-yl)-2-ethoxy-5,6,7,8-tetrahydroquinazolin-4-amine (4): White solid,mp: 263-268°C, yield: 23% (last step),purity: 95% (tR 4.1 min,CH3OH/H2O = 95/5, v/v). 1H NMR (400 MHz,DMSO-d6):δ7.65 (d,1H,J= 8.8 MHz),6.99 (d,1H,J= 1.2 MHz),6.82 (dd,1H,J= 8.8,1.8 MHz),4.29 (q,2H, J= 7.0 MHz),4.01 (s,3H),3.35 (s,3H),2.61-2.56 (m,5H),1.64 (t,2H, J= 5.9 MHz),1.58-1.50 (m,2H),1.36-1.28 (m,5H); HRMS (ESI)m/z calcd. for C20H26N5O [M+H]+: 352.2137,found: 352.2224. 2.2. Kinase enzyme assays
For the kinase modulatory assay,glutathione-S-transferase (GST) fusion enzymes were purchased from Millipore (c-KIT) or Carna (VEGFR-2,PDGFR-&bate;and FGFR-1) and were used without further purification. Ultrapure ATP and DMSO were purchased from Sigma. FAM labeled,fluorescent peptide substrates were synthesized by GL Biochem. Compounds were prepared with DMSO at a final concentration of 2% in the assay system. Assay mixtures were prepared with a 5uL addition of synthesized compounds,followed by 10uL of assay buffer containing enzymes and initiated by adding 10mL assay buffer (50 mmol/L HEPES,10 mmol/L MgCl2,0.0015% Brij-35,and 2 mmol/L DTT) containing ATP and substrate. All kinase reactions were carried out at 3mmol/L peptide substrate andKmof ATP for each kinase and final enzyme concentrations were varied for each target (10 nmol/L PDGFR-&bate;,4 nmol/L FGFR-1,1.2 nmol/L VEGFR-2 and 6 nmol/L c-KIT). The reaction mixtures were incubated at 28°Cfor 1-2 h depending on kinase activity. The reactions were terminated by the addition of 25uL of stop buffer (100 mmol/L HEPES pH 7.5,0.015% Brij-35,0.2% Caliper coating reagent and 50 mmol/L EDTA). Reaction products were separated on the Caliper instrument according to the manufacturer’s instructions. Percent inhibition and percent activation were calculated by using the following equations.
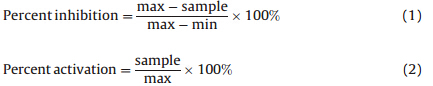
To calculate the half-maximal inhibitory concentration (IC50) and the half-maximal activation concentration (EC50),the percent of remaining kinase activity is plotted against the compound concentration,and the data were fitted using the GraphPad 5.0 software. The accuracy of thein vitro enzyme assays was confirmed by the IC50value of the positive inhibitor,staurosporine, which is in compliance with the reported value. 3. Results and discussion
3.1. In vitro FGFR-1 modulatory activity
The modulatory activities of the target compounds against FGFR-1 were determined using the caliper mobility shift assay. Pazopanib was used as the reference drug. Some preliminary structure-activity relationships (SARs) can be drawn here. As compared with pazopanib,cyclization of R3 and R4(Table 1) into an aliphatic rings,i.e.,cyclopentyl (2a-2i) and cyclohexyl (3a-3g) rings,almost abolished the FGFR-1 inhibitory potency of the resulting compounds,with the exceptions of 2c,3a,and 3b. Surprisingly,eleven analogs showed good activation effects on FGFR-1. A subtle interplay between cycloalkyl size and two substitutions (R2 and R5) seemed to be critical for inhibition/ activation selectivity,however,the R1 substitution effect was limited (2a vs.2b). When R2 substitution is hydrogen,analogs (2c, 2e,and 3b) showed,exclusively,the inhibition profile. Compared with cyclopentyl ring,the cyclohexyl ring is optimal and significantly improves the activation effects (3d vs.2g,3c vs. 2d,3e vs.2h,and 3f vs.2i). Malogen (2h-2iand 3e-3f)ormultiplehalogen (3g) substitutions on the phenyl ring (R5) substantially increase the activation effects of the analogs. Replacement of the 2-aniline substituent with an ethoxy group cannot be tolerated (4),leading to a complete loss of activity. Remarkably,the activation effects of analogs 3d and 3g reach double-digit, nanomolar activities with EC50 values of 68 nmol/L and 99 nmol/L,respectively (Fig. 2). To the best of our knowledge, these two compounds are the most potent,small molecule FGFR-1 activators reported to date. The molecular mechanism of the activation effect has been speculated to be allosteric behavior induced by the FGFR-1 activator. The allosteric binding site and allosteric mechanism on FGFR-1 is under investigation in our laboratories.

|
Download:
|
| Fig. 2. Concentration dependence of FGFR-1 activation effects by3d(A) and3g(B),the concentration of ATP was kept constant at 262mmol/L,while the concentration of compounds3dand3granged from 0.05 nmol/L to 333 nmol/L and 0.19 nmol/L to 12500 nmol/L,respectively. | |
| Table 1 In vitro FGFR-1 modulatory effects of cycloalkyl[d]pyrimidine analogs |
One of the great challenges in kinase ligand discovery is the design of small-molecule modulators with a selectivity profile. To further investigate the specificity of these FGFR-1 activators discovered and,reported herein,we thus tested the modulatory effects of the eleven FGFR-1 activators against other three kinase receptors,including VEGFR-2,PDGFR-&bate;,and c-KIT. The results showed that all FGFR-1 activators had no inhibition or activation effects on VEGFR-2,PDGFR-&bate;,and c-KIT (Table 2). These results suggested,to a certain extent,that these activators might be kinase-selective activators of FGFR-1.
| Table 2 In vitro kinase selectivity profiles of cycloalkyl[d]pyrimidine analogs |
In summary,inspired by the structure of pazopanib (1),two types of novel,cycloalkyl[d]pyrimidine skeletons (2-3) derived from the pyrimidine linker of pazopanib were designed. A total of 16 analogs containing these two skeletons were synthesized,and tested in four pro-angiogenic,kinase inhibitory assays (FGFR-1, VEGFR-2,PDGFR-&bate;,and c-KIT). Unexpectedly,11 analogs were found to show good kinase-selective activation effects on FGFR-1, and two analogs (3d and 3g) showed double-digit,nanomolar selective activation effects on FGFR-1,however,the precise molecular mechanism of the activation effect still needs further investigation. To the best of our knowledge,these activators are the first examples of small-molecular FGFR-1 activators,which might be used as effective chemical probes for the study of FGFR-1 related signaling pathways. Further exploration of the functions of these new activators is under investigation in our laboratories. Acknowledgments
Financial support of this research provided by the National Natural Science Foundation of China (Nos. 21222211,21372001, 91313303),the Program for New Century Excellent Talents in University (No. NCET-12-0853),and the Fundamental Research Funds for the Central Universities are gratefully acknowledged.
| [1] | D.E. Johnson, L.T. Williams, Structural and functional diversity in the FGF receptor multigene family, Adv. Cancer Res. 60 (1993) 1-41. |
| [2] | P.L. Lee, D.E. Johnson, L.S. Cousens, V.A. Fried, L.T. Williams, Purification and complementary DNA cloning of a receptor for basic fibroblast growth factor, Science 245 (1989) 57-59. |
| [3] | V.P. Eswarakumar, I. Lax, J. Schlessinger, Cellular signaling by fibroblast growth factor receptors, Cytokine Growth Factor Rev. 16 (2005) 139-149. |
| [4] | N. Turner, R. Grose, Fibroblast growth factor signalling: from development to cancer, Nat. Rev. Cancer 10 (2010) 116-129. |
| [5] | I. Ahmad, T. Iwata, H.Y. Leung, Mechanisms of FGFR-mediated carcinogenesis, Biochim. Biophys. Acta 1823 (2012) 850-860. |
| [6] | H.K. Ho, A.H.L. Yeo, T.S. Kang, B.T. Chua, Current strategies for inhibiting FGFR activities in clinical applications: opportunities, challenges and toxicological considerations, Drug Discov. Today 19 (2014) 51-62. |
| [7] | V.D. Acevedo, R.D. Gangula, K.W. Freeman, et al., Inducible FGFR-1 activation leads to irreversible prostate adenocarcinoma and an epithelial-to-mesenchymal transition, Cancer Cell 12 (2007) 559-6571. |
| [8] | P.U. Magnusson, A. Dimberg, S. Mellberg, A. Lukinius, L. Claesson-Welsh, FGFR-1 regulates angiogenesis through cytokines interleukin-4 and pleiotrophin, Blood 110 (2007) 4214-4222. |
| [9] | P.A. Harris, A. Boloor, M. Cheung, et al., Discovery of 5-[[4-[(2,3-dimethyl-2Hindazol-6-yl)methylamino]-2-pyrimidinyl]amino]-2-methyl-benzenesulfonamide (Pazopanib), a novel and potent vascular endothelial growth factor receptor inhibitor, J. Med. Chem. 51 (2008) 4632-4640. |






