b State Key Laboratory of Coordination Chemistry, School of Chemistry and Chemical Engineering, Nanjing University, Nanjing 210093, China
1. Introduction
Macroscopically polar-ordered materials are of second-order nonlinear optical activity,piezoelectricity,ferroelectricity,pyroelectricity, and triboluminescence [1, 2, 3, 4, 5, 6],among which ferroelectric behaviors are particularly desirable for applications in telecommunications,optical storage,and information processing [7, 8, 9]. In recent years,developing ferroelectric inorganic compounds such as KH2PO4 (KDP),BaTiO3,and LiNbO3 has been spotlighted [10]. However,ferroelectric materials based on metal- organic coordination compounds (MOCCs) [11, 12, 13] have seldom been referenced. The chemistry of organically templated metal sulfates has received increasing attention over the last few years,of which double sulfates of trivalent metals with monovalent aliphatic ammonium cations are especially intriguing owing to their ferroelastic and ferroelectric properties [14]. Recently,Naïli et al. reported a series of noncentrosymmetric metal sulfates [R-C5H14N2][ MII(H2O)6](SO4)2 and [S-C5H14N2][MII(H2O)6](SO4)2 (MII = Mn,Fe,Co and Ni) by either R-2-methylpiperazine or S-2- methylpiperazine [15],but their temperature-independent dielectric and ferroelectric properties were not further studied. To this end,homochiral (R)-2-methylpiperazine and cobalt sulfate heptahydrate were reacted by the solution method in the presence of concentrated sulfuric acid (H2SO4),yielding a novel Co(II) compound (C5H14N2)[Co(H2O)6](SO4)2 (2) as shown in Scheme 1. The single crystals show 2 and 1 (crystal structure of 1 has been reported by the Naı¨li group) are polymorphs with distinctively different structures. Compounds 1 and 2 crystallize in chiral space group P21,which is associated with point group C2,one of the 10 polar point groups required for ferroelectric behaviors. Experimental results indicate that 1 is ferroelectric. Herein we report the synthesis and crystal structure of 2 as well as the ferroelectric and dielectric properties of the two Co(II) sulfates (1 and 2).
|
Download:
|
| Scheme 1.Preparation of compounds 1 and 2. | |
2. Experimental
Materials and measurements: The start materials and reagents used throughout the experiments were of analytical grade from commercial sources and were used without any further purification. IR spectra were recorded on an IR-Prestige-21 by SHIMADZU CORPORATION in KBr discs in the 400-4000 cm-1 region. Elemental analyses were taken on a Perkin-Elmer 240C elemental analyzer. An electric hysteresis loop of the crystal sample was observed by the Tester Precision Premier II made by Radiant Technologies at room temperature. The powdered sample of the compound was used in a Rigaku SA-HFM3 to produce the X-ray powder diffraction pattern. The compound permittivity (ε = ε' - ε") and dielectric dissipation factor D (D = tan ξ = ε"/ε') were measured on a Tonghui TH2828A with frequencies from 1 kHz to 1 MHz. A pellet sample was made at the high-pressure of 8 MPa.
Synthesis of 1: A mixture of (R)-2-methylpiperazine (10 mmol, 1 g) and CoSO4·7H2O (10 mmol,2.81 g) was mixed with an aqueous solution (20 mL),and then 0.5 mL concentrated sulfuric acid (H2SO4,98%) was added dropwise after stirring the solution for 20 min in air. The reaction mixture solution was evaporated slowly at room temperature for 3 days; pink block crystals were obtained in 56% yield (based on Co). The IR spectra of compound 1 are as follows: 3431(s),3249(m),2885(m),2790(m),2727(m), 1632(m),1612(m),1453(m),1394(m),1073 (s),979(m),606(m). Anal. calcd. for C5H26N2CoO14S2: C,13.02; H,5.68; N,6.07; Found: C,13.12; H,5.71; N,6.12.
Synthesis of 2: A mixture of (R)-2-methyl-piperazine (10 mmol, 1 g) and CoSO4·7H2O (10 mmol,2.81 g) was mixed with an aqueous solution (20 mL),and then 0.5 mL concentrated sulfuric acid (H2SO4,98%) was added dropwise after stirring the solution for 20 min in air. The reaction mixture solution was evaporated slowly at 35 ℃ for 3 days; red block crystals were obtained in 62% yield (based on Co). The IR spectra of compound 2 are as follows: 3372(s),2731(m),2471(m),1629(m),1448(m),1073 (s),979(m), 631(m). Anal. calcd. for C5H26N2CoO14S2: C,13.02; H,5.68; N,6.07; Found: C,13.14; H,5.75; N,6.10.
Single-crystal X-ray diffraction measurements: Single-crystal data were collected at 293(2) K on a Rigaku SCXmini CCD diffractometer equipped with graphite-monochromated Mo Ka radiation (l = 0.71073A˚ ). The structure was solved using direct methods and successive Fourier difference synthesis (SHELXS-97) [16] and refined using the full-matrix least-squares method on F2 with anisotropic thermal parameters for all nonhydrogen atoms (SHELXL-97) [17] All non-H atoms and H atoms were refined anisotropically. All H atoms were placed in geometrically idealized positions and constrained to ride on their parent atoms with Uiso(H) = 1.2Ueq. Detailed information about the crystal data and structure determination for the title compounds is summarized in Table 1. Selected bond distances and angles are given in Table S1 and Table S2 in Supporting information. Detailed information about hydrogen bonds of compounds 1 and 2 are given in Table S3 and Table S4 in Supporting information. CCDC-926566 for 1 and CCDC-926567 for 2.
| Table 1 Crystallographic data and details of refinements for 1 and 2. |
3. Results and discussion 3.1. IR
The IR spectra display typical broad vibration peak of SO4 2- between 1100 cm-1 and 1050 cm-1,verifying the existence of SO4 2-. The broad band at around 3200-3500 cm-1 corresponds to the n(O-H) stretching frequency of coordinated water molecules (Fig. S1 and Fig. S2 in Supporting information).
3.2. Crystal structure of 1 and 2
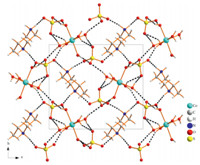
Compound 2 crystallizes in chiral space group P21,and the asymmetric unit of 2 contains transition metal Co2+ cations octahedrally coordinated by six water molecules,[Co(H2O)6]2+=,isolated sulfate anions SO4 2-,and diprotonated homochiral 2-methylpiperazine moieties C5H14N2 2+. Fig. 1 shows that the six-membered ring of piperazine in the asymmetric unit of 2 has a chair conformation. Many H-bonds were found between the H atom of protonated NH2 group and water,water and the N atom of piperazine ring,and water and water,thus forming a threedimensional (3D) framework through H-bonds (Fig. 2).

|
Download:
|
| Fig. 1.Asymmetric unit representation of 2. | |
|
Download:
|
| Fig. 2.Perspective views 3D framework of 2 through H-bonding along the a-axis. | |
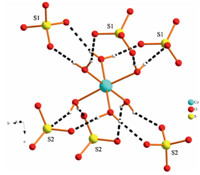
The local coordination environment around the [Co(H2O)6]2+ cations in 2 is shown in Fig. 3. The octahedral environments of Co2+ cations are irregular (Table S2),which is consistent with those of other isostructural metal sulfates [15]. The Co-Ow bond distances range between 2.0708(18) and 2.121(2)A˚ . Compound 2 contains [Co(H2O)6]2+ cations which are stacked along the [1 0 0] and [0 1 0] directions and are separated by SO4 2- anions and [R-C5H14N2]2+ cations. Each [Co(H2O)6]2+ cation donates hydrogen bonds to six adjacent SO4 2- groups,each of which accepts hydrogen bonds from at least two H2O ligands at the same Co center. The [R-C5H14N2]2+ cations also donate hydrogen bonds to adjoining SO4 2- groups,creating an extended supramolecular structure. The cation-anion distances range between 2.678(3) and 2.800(3)A˚ for N-H. . .O interactions and between 2.673(3) and 2.981(3)A˚ for O-H. . .O interactions in 2,respectively (Table S4).
|
Download:
|
| Fig. 3.Neighboring sulfates in the environment of [Co(H2O)6] in 2. | |
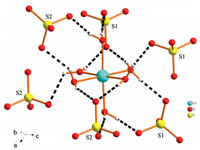
Compound 1 also crystallizes in the chiral space group P21,An asymmetric unit of 1 contains transition metal CoII cations octahedrally coordinated by six water molecules,[Co(H2O)6]2+, isolated sulfate anions,SO4 2-,and diprotonated homochiral 2-methylpiperazine moieties,C5H14N2 2+ (Fig. 4). It is interesting to note that 1 and 2 are polymorphs,however,their hydrogen bonds show obvious difference. The [Co(H2O)6]2+ cations in 1 donate hydrogen bonds to six adjacent SO4 2- tetrahedra,as shown in Fig. 5. The geometries of these interactions differ from those of compounds 2,as each [Co(H2O)6]2 cation is surrounded by two monodentate,two bidentate and two tridentate (SO4)2 anions. Besides,the [R-C5H14N2]2+ cations in compounds 1 also donate hydrogen bonds to neighboring SO4 2- tetrahedra (Fig. S3 in Supporting infomation). The N-H. . .O distances range between 2.739(4) and 3.046(4)A˚ ,while the O-H. . .O distances range between 2.664(3) and 2.829 (4)A˚ (Table S3).

|
Download:
|
| Fig. 4.Asymmetric unit representation of 1. | |
|
Download:
|
| Fig. 5.Neighboring sulfates in the environment of [Co(H2O)6] in 1. | |
3.3. Powder XRD pattern
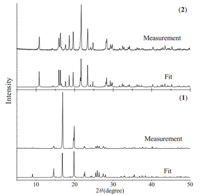
The phase purities of 1 and 2 were determined by X-ray powder diffraction (XRPD) experiments. The peak positions of the experimental and simulated XRPD patterns are in good agreement, which demonstrates that 1 and 2 were obtained successfully as pure crystalline phases (Fig. 6). The differences in intensity may be attributed to the preferred orientation of the powder sample.
|
Download:
|
| Fig. 6.Difference of XRD pattern of the powdered sample between 1 and 2, where the lower line is single crystal diffraction pattern. | |
3.4. Ferroelectric
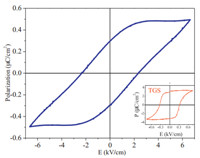
Both 1 and 2 crystallize in chiral space group P21,which is associated with point group C2,one of the 10 polar point groups (C1,C2,Cs,C2n,C4,C4n,C3,C3n,C6,C6n) prerequisite for ferroelectric behaviors. Experimental results exhibit that compound 1 is ferroelectric (Fig. 7),the coercive electric field (Ec) of which reaches 2.35 kV/cm,which is higher than that of triglycine sulphate (TGS) (0.3 kV/cm). The remnant polarization (Pr) and spontaneous polarization (Ps) of 1 are about 0.3 and 0.5 mC/cm2, respectively,which are lower than those of TGS (3.5 mC/cm2) and higher than those of Rochelle salt (0.25 mC/cm2). In contrast,2 does not present any electric hysteresis loops. Despite being polymorphs, the crystal structures of 1 and 2 differ significantly. The angles of S-Co-S (two SO4 2- and Co2+) in 1 and 2 are 60.748 and 32.878,respectively,and their H-bonds are different as well. Differences in the XRD patterns of the powdered samples between 1 and 2 are shown in Fig. 6.
|
Download:
|
| Fig. 7.Polarization versus applied electric field approximately along the b-axis in 1, while the inset is the ferroelectric loop of TGS measured at the same conditions as 1. | |
There are many H-bonds between isolated sulfate anions,six water molecules of [M(H2O)6]2+,and diprotonated homochiral 2-methylpiperazine moieties C5H14N2 2+,thus rendering the ferroelectricity of 1 to be KDP-type ferroelectric. Besides H-bonds, we ascribe the ferroelectricity to the orientational disorder of two chair conformations of the six-membered piperazine ring,which reversibly changes dipole moment correspondingly [18].
3.5. Dielectric property
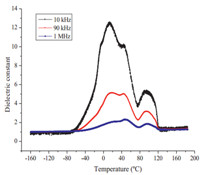
It is well-known that ferroelectric demonstrate anomalous physical properties near the structure phase transition point,such as changes in the dielectric constant [19, 20]. We measured the dielectric changes at different frequencies (10 kHz,90 kHz,and 1 MHz),as shown in Figs. 8 and 9. However,with decreasing frequency,the measurement was vulnerable to external interference, leading to increasingly larger background noise,especially at low frequencies ranging up to 10 kHz. Temperature dependence of the real part (ε') of the dielectric constant of compound 1 is shown in Fig. 8. Upon heating,ε' increases very slowly below -40 ℃ and above 130 ℃,but there are two dielectric anomalies at 23 ℃ and 96 ℃. The dielectric peak at 23 ℃ can be attributed to the structure phase transition which is consistent with the DSC results (Fig. S4 in Supporting information). The second dielectric anomalies at around 96 ℃ can be ascribed the departure of the first one water molecule. In addition,temperature dependence of the real part (ε') of the dielectric constant of compound 2 measured is shown in Fig. 9. Upon heating,ε' increases very slowly below 95 ℃,but there is one dielectric anomaly at 130 ℃ which can be ascribed to the departure of the water molecules being consistent with the TGA results of compound 2.
|
Download:
|
| Fig. 8.The temperature-dependence-permitivity at different frequencies powdered at the low temperature range for compound 1. | |

|
Download:
|
| Fig. 9.The temperature-dependence-permitivity at different frequencies powdered at the low temperature range for compound 2. | |
3.6. Thermal analyses
DSC is one of the thermodynamic methods used to detect if a compound displays reversible phase transition as a function of temperature. Provided structural phase transition and thermal entropy change,heat anomalies can be observed during heating and cooling. Heating the crystalline sample of 1 shows a main endothermic peak at 23.5 ℃ and a main exothermic peak on cooling at 23.1 ℃ (Fig. S4).
The TGA curves of compound 1,between room temperature and 1000 ℃,are shown in Fig. S5 in Supporting information. According to the TG curve the dehydration of the precursor occurs in two stages. First,an immediateweight loss of 3.608% observed at 140 ℃ corresponds to the departure of only one water molecule (calculated weight loss,3.901%). The second stage of the dehydration takes place between 155 and 223 ℃,and corresponds to the loss of the remaining water molecules (observed weight loss,19.218%; calculated weight loss, 19.508%) giving rise to the anhydrous compound [R-C5H14N2] Co(SO4)2. The next transformation starts at 289 ℃ and corresponds to the decomposition of the amine entity and partial decomposition of the sulfate groups leading to the CoSO4 phase (observedweight loss,42.363%; calculatedweight loss,42.933%). The last stage corresponds to the decomposition of the cobalt sulfate,starting at about 772 ℃,and gives rise to the cobalt oxide Co3o4. The TGA curves of compound 2,between room temperature and 1000 ℃,are shown in Fig. S6 in Supporting information. According to the TG curve,the dehydration of the precursor occurs in two stages. First,an immediate weight loss of 3.608% observed between 86 and 119 ℃ corresponds to the departure of only one water molecule (calculated weight loss,3.901%). The second stage of the dehydration takes place between 119 and 245 ℃,and corresponds to the loss of the remaining water molecules (observed weight loss,18.815%; calculated weight loss,19.508%) giving rise to the anhydrous compound [R-C5H14N2] Co(SO4)2. The amine decomposition together with loss of one sulfate group [R-C5H14N2] Co(SO4)2 gives rise to the manganese sulfate at 417 ℃ (observed weight loss,42.932%; theoretical,43.308%). The last stage corresponds to the decomposition of the cobalt sulfate,starting at about 782 ℃,and gives rise to the cobalt oxide Co3o4.
4. Conclusion
The present work has demonstrated that homochiral 2- methylpiperazine,as a template,cooperates with 3d transition metal (Co2+) sulfates to pave an innovative way for the exploration of metal-organic ferroelectrics.
Acknowledgments
This work was funded by the National Natural Science Foundation of China (No. 21201087),Jiangsu Province NSF BK20131244 and the Foundation of Jiangsu Educational Committee (No. 11KJB150004) and sponsored by Qing Lan Project of Jiangsu province. L.-Z. Chen thanks Prof. Xiong Ren-Gen for revising this manuscript. Sponsored by Qing Lan Project of Jiangsu Province and Jiangsu Overseas Research & Training Program for University Prominent Young & Middle-aged Teacher and Presidents.
Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.05.038.
| [1] | (a) W. Zhang, L.Z. Chen, R.G. Xiong, T. Nakamura, S.P.D. Huang, New ferroelectrics based on divalent metal ion alum, J. Am. Chem. Soc. 131 (2009) 12544-12545;(b) W. Zhang, R.G. Xiong, Ferroelectric metal-organic frameworks, Chem. Rev. 112 (2012) 1163-1195. |
| [2] | L.Z. Chen, D.D. Huang, J.Z. Ge, F.M. Wang, A novel Ag(I) coordination polymers based on 2-(pyridin-4-yl)-1H-imidazole-4,5-dicarboxylic acid: syntheses, structures, ferroelectric, dielectric and optical properties, Inorg. Chim. Acta 406 (2013) 95-99. |
| [3] | P.A. Maggard, C.L. Stern, K.R. Poeppelmeier, Understanding the role of helical chains in the formation of noncentrosymmetric solids, J. Am. Chem. Soc. 123 (2001) 7742-7743. |
| [4] | H.L. Cai, T. Zhang, L.Z. Chen, R.G. Xiong, The first homochiral compound with temperature-independence of piezoelectric properties, J. Mater. Chem. 20 (2010) 1868-1870. |
| [5] | H. Zhang, X.M. Wang, K.C. Zhang, B.K. Teo, Molecular and crystal engineering of a new class of inorganic cadmiuμ-thiocyanate polymers with host-guest complexes as organic spacers, controllers, and templates, Coord, Chem. Rev. 183 (1999) 157-195. |
| [6] | L.N. Li, J.X. Ma, C. Song, et al., A 3D polar nanotubular coordination polymer with dynamic structural transformation and ferroelectric and nonlinear-optical properties, Inorg. Chem. 51 (2012) 2438-2442. |
| [7] | W. Eerenstein, N.D. Mathur, J.F. Scott, Multiferroic and magnetoelectric materials, Nature 442 (2006) 759-765. |
| [8] | D.W. Fu, H.L. Cai, Y.M. Liu, et al., Diisopropylammonium bromide is a hightemperature molecular ferroelectric crystal, Science 339 (2013) 425-428. |
| [9] | S. Horiuchi, Y. Tokura, Organic ferroelectrics, Nat. Mater. 7 (2008) 357-366. |
| [10] | J.F. Scott, Applications of modern ferroelectrics, Science 315 (2007) 954-959. |
| [11] | (a) Y. Zhang, W. Zhang, S.H. Li, et al., Ferroelectricity induced by ordering of twisting motion in a molecular rotor, J. Am. Chem. Soc. 134 (2012) 11044-11049;(b) D.W. Fu, Y.M. Song, G.X. Wang, et al., Dielectric anisotropy of a homochiral trinuclear nickel(II) complex, J. Am. Chem. Soc. 129 (2007) 5346-5347. |
| [12] | G.C. Xu, X.M. Ma, Z. Li, Z.M. Wang, S. Gao, Disorder-order ferroelectric transition in the metal formate framework of [NH4][Zn(HCOO)3], J. Am. Chem. Soc. 132 (2010) 9588-9590. |
| [13] | H.X. Zhao, X.J. Kong, H. Li, et al., Transition from one-dimensional water to ferroelectric ice within a supramolecular architecture, Proc. Natl. Acad. Sci. U.S.A. 108 (2011) 3481-3486. |
| [14] | (a) A.N. Holden, B.T. Matthias, W.J. Merz, J.P. Remeika, New class of ferroelectrics, Phys. Rev. 98 (1955) 546;(b) N. Galesic, V.B. Jordanovska, Structures of dimethylammonium metal(III) sulfate hexahydrates (metal=Al, Cr), Acta Crystallogr. Sect. C 48 (1992) 256-258;(c) L.F. Kirpichnikova, L.A. Shuvalov, N.R. Ivanov, B.N. Prasolov, E.F. Andreyev, Ferroelectricity in the dimethylaminaluminiuμ-sulphate crystal, Ferroelectrics 96 (1989) 313-317. |
| [15] | F. Hajlaoui, H. Naïli, S. Yahyaoui, et al., Synthesis, crystal structures and thermal behaviour of organic-inorganic hybrids incorporating a chiral diamine, J. Organomet. Chem. 700 (2012) 110-116. |
| [16] | G.M. Sheldrick, SHELXS-97, in: Program for Crystal Structure Solution, University of Göttingen, Germany, 1997. |
| [17] | G.M. Sheldrick, SHELXL-97, in: Program for Crystal Structure Refinement, University of Göttingen, Germany, 1997. |
| [18] | T. Zhang, L.Z. Chen, M. Gou, et al., Ferroelectric homochiral organic molecular crystals, Cryst. Growth Des. 10 (2010) 1025-1027. |
| [19] | (a) Z.H. Sun, T.L. Chen, J.H. Luo, M.C. Hong, Bis(imidazolium) L-tartrate: a hydrogen-bonded displacive-type molecular ferroelectric material, Angew. Chem. Int. Ed. 51 (2012) 3871-3876;(b) D.W. Fu, W. Zhang, H.L. Cai, et al., A multiferroic perdeutero metal-organic framework, Angew. Chem. Int. Ed. 50 (2011) 11947-11951. |
| [20] | (a) L.Z. Chen, H. Zhao, J.Z. Ge, R.G. Xiong, H.W. Hu, Observation of deuteration effect in Co-crystal system: hexame-thylenetetraminium 3,5-dinitrobenzoate hemideuterated water, Cryst. Growth Des. 9 (2009) 3828-3831;(b) L.Z. Chen, D.D. Huang, J.Z. Ge, F.M. Wang, Temperature-induced reversible structural phase transition of 1-(chloromethyl)-1,4-diazoniabicyclo[2.2.2]octane bis(perchlorate), CrystEngComm 16 (2014) 2944-2949;(c) L.Z. Chen, D.D. Huang, Synthesis, structure and dielectric properties of a novel Gd coordination polymer based on 2-(pyridin-4-yl)-1H-imidazole-4,5-dicarboxylate, Chin. Chem. Lett. 25 (2014) 279-282. |