b School of Chemical Engineering and Environment, Weifang University of Science and Technology, Weifang 262700, China
1. Introduction
TiO2-based materials are attracting increased attention because of their well-ordered porous structures and large surface areas originating from the silica support,which provide nanoscale reaction vessels,condensation effects,high adsorptive capacities, and high dispersivities of active species [1, 2]. The rising interest in engineering the morphology of semiconducting materials over the past decade has also led to a focus on the effects of electronic structure and shape on the performance of TiO2-based devices. Accordingly,research on the intrinsic morphology-property relationship has elicited an urgent need for adjustable synthetic strategies that can enable the precise control of the morphology of materials with designed functionalities [3]. Unfortunately,few studies have reported on the effects of varied aspect ratios (ARs) on the photocatalytic activity of titanosilicate composites. Therefore, the synthesis of titanosilicate composites with tunable AR is highly significant.
Compared with the flexible synthesis of silicates [4, 5, 6],most procedures for the conventional synthesis of titania-based rods with varied ARs rely on hard templates,such as silica-based nanomaterials [7],a-Fe2O3 ellipsoids [8],and calcite [9]. In this paper,we report an easy soft template-based approach to prepare rod-shaped mesoporous titanosilicate composites (RMTSs) with controllable ARs by adjusting the surfactant concentration,catalyst amount,and stirring rate at room temperature. The effects of length and width on the photocatalytic activity of the products were also studied. The results can serve as a foundation for the large-scale fabrication of nanoscale devices with various applications.
2. Experimental
Highly ordered 2-D hexagonal RMTSs were synthesized using an easy method at room temperature according to the molar ratios listed in Table 1. Rods with AR = 4 and Ti/Si = 0.5 were typically prepared by first dissolving 0.28 g of cetyltrimethylammonium bromide (CTAB) in 34.82 g of H2O under moderate heat to form a homogeneous solution. After the solution was cooled to 22 ℃, 1.38 mL of ammonium hydroxide (NH4OH,26 wt%) was introduced, and the mixture was stirred for 1-1.5 h at a stirring rate of 250 rpm. About 0.56 g of tetraethyl orthosilicate (TEOS) was added dropwise into this mixture under vigorous stirring,followed by the addition of 0.46 g of tetrabutyl titanate (TBT) at an injection rate of 1 mL/min in 0.5 h of stirring at a constant rate of 500 rpm. The stirring speed was then adjusted to 250 rpm,and stirring was continued for another 4 h. The mixture was placed into a Teflon autoclave and heated at 100 ℃ for 24 h. The solid products were collected by filtration and were washed and dried at room temperature,and the resultant powder was calcined at 450 ℃ for 5 h. The obtained RMTS products were named ARn-s-r,where n,s, and r represent the AR,stirring rate,and Ti/Si molar ratio, respectively.
| Table 1 RMTS prepared from different stirring rate and reactant molar composition and their physical characterization. |
3. Results and discussion 3.1. Physical characterization of RMTSs
Prior to any other analyses,the titanium amount actually present in the calcined titanosilicate materials was measured by energy-dispersive X-ray spectroscopy (EDS). However,the determined molar ratio (0.45-0.49) of Ti/Si in calcined titanosilicate materials was lower than that (0.50) in the starting solution. This finding meant that a portion of the titanium was lost during the extraction step [10]. The morphologies of mesoporous materials are influenced by the free energy change,as well as other interactions such as shearing force that depends on the stirring rate. To understand the effects of shearing force on AR,the stirring rate was increased from 150 rpm to 350 rpm while the CTAB concentration was kept constant. At 150 rpm stirring rate,the length of the sample obtained was 640 nm (Fig. 1d),resulting in an AR of 5. The length decreased with an increased stirring rate of 500 rpm (Fig. 1a). The decrease in rod length with increased stirring rates indicated that stirring influenced the longitudinal growth of the reaction particles by controlling the flow direction of the solution [11]. High-resolution transmission electron microscopy (HRTEM) images of AR2-350-0.5 and AR4-250-0.5 are shown in Fig. 2. The HRTEM images of both AR2-350-0.5 and AR4-250-0.5 clearly showed well-ordered parallel straight mesochannels arraying along the long axis; the pores were uniform and had an average diameter of 3.85 nm; the pore wall thicknesses (Fig. 2) were about 0.80 nm. Some anatase TiO2 (<3 nm) can also be observed in the pore walls. The average d-spacing of the lattice fringes was about 0.35 nm,agreeing with the d1 0 1 value of anatase TiO2.

|
Download:
|
| Fig. 1.SEM images of RMTSs with regulated length synthesized by adjusting the stirring speed: (a) AR1-500-0.5,(b) AR2-350-0.5,(c) AR4-250-0.5,and (d) AR5-150-0.5. | |

|
Download:
|
| Fig. 2.HRTEM images of RMTSs with regulated length synthesized by adjusting the stirring speed: (a and b) AR2-350-0.5 and (c and d) AR4-250-0.5. | |
Fig. S1A in Supporting information shows the variations in SAXRD patterns with varied lengths. A strong feature at a low angle ((1 0 0) reflection line) and two other weaker peaks at higher angles ((1 1 0) and (2 0 0) reflection lines) were observed. This finding indicated that all samples were hexagonal mesoporous materials [12, 13]. The mesoporous parameters deduced from the XRD patterns (Table 1) revealed that the plane distance (d1 0 0) of the sample with varied ARs did not change,which showed that stirring did not affect the hexagonal structure of the materials. Using the Bragg’s rule (a0 = 2d1 0 0/30.5),the unit cell dimension (a0) was deduced as about 4.50 nm. Except for a large peak at around 2u = 23°,which was characteristic of amorphous silica,no peaks belong to titanium existed in any of the samples (Fig. S1B in Supporting information). The lack of peaks indicated the incorporation of Ti species into the silica framework [10] and the existence of small poorly crystalline agglomerates of TiO2 (<3 nm) [14]. Xray photoelectron spectroscopy (XPS) was used to investigate the presence of titanium. Fig. S2A in supporting information shows the Ti (2p) XPS spectra of both AR2-350-0.5 and AR4-250-0.5,and three peaks centered at 458.5,459.6,and 464.5 eV were observed. Among them,the positions of the 458.5 eV (Ti 2p3/2) and 464.3 eV (Ti 2p1/2) were characteristic of anatase TiO2,which was consistent with the HRTEM image. The Ti 2p3/2 regions showed a doublet at 458.5 and 459.6 eV (Fig. S2A),corresponding to a tetrahedral geometry of titanium and indicative of its integration into the silica framework [10]. Thus,we can conclude that a portion of titanium was incorporated into the skeleton of mesoporous SiO2. Fig. S2B in Supporting information shows the O (1s) XPS spectra of AR2-350- 0.5 and AR4-250-0.5. One peak was centered at 531.2 eV,which was characteristic of oxygen in the Si-O-Ti linkage. This finding further confirmed that titanium ions were incorporated into the silica framework [15].
Fig. S3 in Supporting information shows a typical reversible type-IV adsorption isotherm for the RMTSs [16]. Compared with the largest surface area (536.92 m2/g) of AR4-250-0.5,a high AR value of 5 (AR5-150-0.5) did not result in a corresponding increase in specific surface area,which may be due to the fact that higher ARs were inconducive to the effective adsorption of N2 and other materials [5, 17]. Taking into account the measurement errors,the pore diameter was deemed constant at 4 nm,indicating that growth on the longitude by stirring did not contribute to the growth of pore size. The pore wall thickness (Tp) was about 0.50 nm,which supported the HRTEM image.
The influence of surfactant concentration on AR was analyzed by increasing the CTAB concentration from 5.50 mmol/L to 22.00 mmol/L. Rods with fixed lengths varied from 105 nm to 450 nm and ARs from 1 to 4 were obtained (Fig. 3) depending on the concentration of the reagents used. These findings indicated that the hexagonal arrays were significantly altered by minimal changes in CTAB concentration [18]. For CTAB concentrations of 5.50,11.00,and 22.00 mmol/L,the products showed lengths of 105,180,and 450 nm,respectively (Fig. 3). Rod width could be controlled by adjusting the NH4OH concentration in the reaction mixture,and higher concentrations of NH4OH led to larger widths [5, 19, 20]. In the present work,the product width increased from 75 nm to 120 nm with increased amount of NH4OH from 0.69 mL to 1.38 mL. Under these reaction conditions,the highest NH4OH concentration resulted in particles with the largest width of approximately 120 nm. The N2 adsorption-desorption isotherms and BJH pore size distribution curves of the samples are shown in Fig. S4 in Supporting information. Previous reports have indicated that the pore size slightly changed by altering only the surfactant concentration within a small range [21, 22]. Thus,the increase in pore size (Fig. S4 and Table 1) can be reasonably regarded as the effect of NH4OH [23].

|
Download:
|
| Fig. 3.TEM images of RMTSs with regulated ARs: (a) AR1-250-0.5,(b) AR2-250-0.5,and (c) AR4-250-0.5.F.-J. Chen et al. / Chinese Chemical Letters 964 25 (2014) 962-966 | |
Literature reports indicate that the CTAB concentration mainly impacts the longitudinal growth (length-controlling process) of products,whereas the NH4OH amount affects their growth in the latitudinal direction. The morphological transformation of Ti nanoparticles with increased CTAB has also been extensively studied [18, 24, 25]. However,little work has been done to analyze the changes in length and width of resultant particles within a small concentration range. These findings advance the understanding on self-assembled surfactants and enable the flexible design and construction of new functional nanomaterials.
3.2. Mechanism
Scheme 1 summarizes the possible growth processes achieved by slight changes in stirring rate and reactant concentration based on morphological analyses. By keeping the stirring rate constant, mesoporous titanosilicate materials with rod-shaped structure are formed by the compromise between the quenching disorder provided by the inorganic precursors and the elastic force,which minimizes the distortions of the rod-like hexagonal array [26].

|
Download:
|
| Scheme 1.Formation mechanism of the mesoporous titanosilicate composites with tunable ARs through soft template method by using CTAB as a template and NH4OH as catalyst. | |
Route A shows that the length of the rod-like hexagonal array increases with decreasing shear force caused by stirring,which suggests that stirring influences the orientation of the reaction particles by controlling the solution flow direction [12,1°,19]. At lower stirring rates,the molecular assemblies become more uniform and induce longitudinal growth. Thus,higher ARs are maintained,such as in the case of AR5-150-0.5 (Fig. 1d). By contrast,higher stirring rates result in less molecular assembly disorder in terms of length but facilitate the transverse growth of mesoporous rods with different lengths. This hypothesis is consistent with the findings in the SEM images (Fig. 1a). Route B shows that longitudinal growth takes place by further addition of CTAB. The local orientation order is presumed constant because the distortions induced by the inorganic precursors have spatial ranges much larger than the molecular size,resulting in ARs ranging from 1 to 4 (Fig. 3). Excess amounts of inorganic precursors result in elastic forces,induced by CTAB,inadequate to antagonize the disorder provided by the inorganic precursors and result in AR dispersion. This dispersion causes the uneven lengths and widths observed in AR1-250-0.5 (Fig. 3a). The correlation length is a key parameter in understanding phase changes in the presence of disorder.
Subsequently,effects of NH4OH on the latitude,as outlined in Route C,involve: (1) in order to balance the charge,the thickness of the counterion layer between micelle and OH-1 may increase to keep the total OH-1 constant,resulting in the enlargement of pore size; (2) NH4OH variation may also change the electrostatic charge distribution,affecting the interactions of the head groups in the micelle and resulting in the expansion of the micelle. That gives rise to the increased widths of rod-like hexagonal arrays; (3) the increased hydrolysis and polymerization rates of inorganic sources may make inorganic substances attach more to the rod-like hexagonal arrays [20],which contribute to the increase of width. In short,the width of the rods increases as the NH4OH concentration increases.
3.3. Photocatalytic activity studies
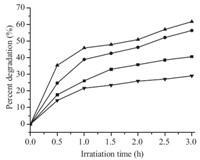
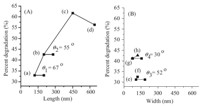
Fig. 4 shows the percentage of p-chlorophenol degradation under UV light irradiation over the Ti-loaded mesoporous photocatalysts. AR affects the photocatalytic activity of the rodshaped mesoporous titanosilicate composites. Among the samples, AR4-250-0.5 was the most active one,showing a percent degradation of 62.00% under the given reaction conditions. The photocatalytic activity of the catalysts increases with increased ARs at lower AR values,which agrees with a previous report [27]. Increasing the AR of nanoparticles (e.g.,AR5-150-0.5) does not show a corresponding increase in catalytic efficiency. AR5-150-0.5 likely features a relatively low surface area (425.0 m2/g) and high AR,both of which inhibit the contact between p-chlorophenol and the catalyst [17]. Fig. S5 in Supporting information shows that the photocatalytic efficiency of the products increased with longer reaction time. Among the samples,AR4-250-2 was the most active one,showing a photocatalytic efficiency of 97.67%. This efficiency is attributed to its large BET surface area and relatively high titanium dioxide content (354.10 m2/g and Ti/Si = 2,respectively). To analyze the various factors contributing to the photocatalytic activity of RMTSs further,we discussed the effects of length and width in Fig. 5. These parameters are positively related to the photocatalytic activity at lower ARs. Fig. 5A shows the photocatalytic activity of titanosilicate materials with different lengths synthesized by adjusting the stirring rate. The angles (u) of the line with X-axis (length) are 67° and 55°,while the angles of line with X-axis (width) are 52° and 30° (Fig. 5B),respectively. Thus,the length of RMTSs may be reasonably deduced to be more important in increasing photocatalytic activity than their width; longitudinal growth in this case appears to be a more dominant factor than the increase in BET surface area,which is one of the determinants of photocatalytic efficiency (Fig. S6 in Supporting information).
|
Download:
|
Fig. 4.Photocatalytic percent degradation of p-chlorophenol in the presence of AR1-250-0.5 as a function of UV light irradiation time ( ),AR2-250-0.5 (■),AR4-250-0.5 (▲),and AR5-150-0.5 (●). ),AR2-250-0.5 (■),AR4-250-0.5 (▲),and AR5-150-0.5 (●).
|
|
|
Download:
|
| Fig. 5.(A) Impact of length on photocatalytic percent decomposition in the presence of (a) AR1-500-0.5,(b) AR2-350-0.5,(c) AR4-250-0.5,and (d) AR5-150-0.5. (B) Impact of the width on photocatalytic percent decomposition in the presence of (e) AR1-250-0.5,(f) AR1-500-0.5,(g) AR2-250-0.5,and (h) AR2-350-0.5. | |
4. Conclusion
RMTSs with controllable ARs were synthesized at very low surfactant concentrations under continuous stirring. Three routes were used to effectively control the AR. Among these routes, increasing the CTAB concentration and adjusting the stirring rate were found to mainly contribute to the longitudinal growth of RMTSs,resulting in ARs ranging from 1 to 4. However,only stirring can effectively promote growth to AR = 5. NH4OH influenced the hydrolysis and polymerization of the inorganic sources,as well as the hexagonal array aggregation behaviors. Thus,NH4OH affected the growth in the latitudinal direction,and an increased width from 75 nm to 120 nm was observed with increased amount of NH4OH in the reaction system. Among the obtained samples,AR4- 250-2 was the most active,showing 97.67% p-chlorophenol degradation under the given reaction conditions. This activity was attributed to its large BET surface area (354.10 m2/g). Photocatalytic activity data of different lengths and widths showed that both parameters are positively related to photocatalytic activity at lower AR. However,the length of RMTSs played a greater role in the photocatalytic activity of the resultant products than their width. The results of this work provided a foundation for the large-scale fabrication of nanoscale devices.
Acknowledgments
This work was supported by the National Natural Science Foundation of China (Nos. 20976100,51372124),the Natural Science Foundation of Shandong Province (Nos. ZR2010BM013, ZR2011BQ009),the Program for Scientific Research Innovation Team in Colleges and Universities of Shandong Province (No. 201207),and Key Laboratory of Colloid and Surface Chemistry, Ministry of Education (Shandong University,No. 201205).
Appendix A. Supplementary data
Supplementary data associated with this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.05.034.
| [1] | Y. Pu, G.L. Zhu, B.S. Ge, et al., Photocurrent generation by recombinant allophycocyanin trimer multilayer on TiO2 electrode, Chin. Chem. Lett. 24 (2013) 163-166. |
| [2] | S. Abdolmohammadi, Simple route to indeno [1,2-b] quinoline derivatives via a coupling reaction catalyzed by TiO2 nanoparticles, Chin. Chem. Lett. 24 (2013) 318-320. |
| [3] | W. Li, Z. Wu, J. Wang, A.A. Elzatahry, D. Zhao, A perspective on mesoporous TiO2 materials, Chem. Mater. 26 (2014) 289-298. |
| [4] | T. Lin, X. Zhang, R. Li, T. Bai, S.Y. Yang, Synergistic catalysis of isolated Fe3+ and Fe2O3 on FeOx/HZSM-5 catalysts for Friedel-Crafts benzylation of benzene, Chin. Chem. Lett. 22 (2011) 639-642. |
| [5] | N. Murakami, S. Katayama, M. Nakamura, T. Tsubota, T. Ohno, Dependence of photocatalytic activity on aspect ratio of shape-controlled rutile titanium (IV) oxide nanorods, J. Phys. Chem. C 115 (2010) 419-424. |
| [6] | X.J. Wu, Y.Y. Jiang, D.S. Xu, A unique transformation route for synthesis of rodlike hollow mesoporous silica particles, J. Phys. Chem. C 115 (2011) 11342-11347. |
| [7] | W. Li, Y.H. Deng, Z.X. Wu, et al., Hydrothermal etching assisted crystallization: a facile route to functional yolk-shell titanate microspheres with ultrathin nanosheets-assembled double shells, J. Am. Chem. Soc. 133 (2011) 15830-15833. |
| [8] | W. Li, J.P. Yang, Z.X. Wu, et al., A versatile kinetics-controlled coating method to construct uniform porous TiO2 shells for multifunctional core-shell structures, J. Am. Chem. Soc. 134 (2012) 11864-11867. |
| [9] | D.X. Liu, M.Z. Yates, Fabrication of size-tunable TiO2 tubes using rod-shaped calcite templates, Langmuir 23 (2007) 10333-10341. |
| [10] | K. Zimny, C. Carteret, M. Stébé, J. Blin, Multitechnique investigation of mesoporous titanosilicate materials prepared from both the self-assembly and the liquid crystal mechanisms, J. Phys. Chem. C 115 (2011) 8684-8692. |
| [11] | H. Jin, Z. Liu, T. Ohsuna, et al., Control of morphology and helicity of chiral mesoporous silica, Adv. Mater. 18 (2006) 593-596. |
| [12] | C. Kresge, M. Leonowicz, W. Roth, J. Vartuli, J. Beck, Ordered mesoporous molecular sieves synthesized by a liquid-crystal template mechanism, Nature 359 (1992) 710-712. |
| [13] | Z.L. Yang, J.L. Li, C.L. Zhang, et al., Two-dimensional mesoporous materials: from fragile coatings to flexible membranes, Chin. Chem. Lett. 24 (2013) 89-92. |
| [14] | Y. Liu, R.O. Claus, Blue light emitting nanosized TiO2 colloids, J. Am. Chem. Soc. 119 (1997) 5273-5274. |
| [15] | G. Li, X. Zhao, Characterization and photocatalytic properties of titaniuμ-containing mesoporous SBA-15, Ind. Eng. Chem. Res. 45 (2006) 3569-3573. |
| [16] | H.M. Abdelaal, Fabrication of hollow silica microspheres utilizing a hydrothermal approach, Chin. Chem. Lett. 25 (2014) 627-629. |
| [17] | G.N. Shao, G. Elineema, D.V. Quang, et al., Two step synthesis of a mesoporous titania-silica composite from titanium oxychloride and sodium silicate, Powder Technol. 217 (2012) 489-496. |
| [18] | N.S.M. Yusof, M.N. Khan, M. Ashokkumar, Characterization of the structural transitions in CTAB micelles using fluorescein isothiocyanate, J. Phys. Chem. C 116 (2012) 15019-15027. |
| [19] | Z. Jin, X. Wang, X. Cui, Synthesis and morphological investigation of ordered SBA-15-type mesoporous silica with an amphiphilic triblock copolymer template under various conditions, Colloids Surf. A 316 (2008) 27-36. |
| [20] | W. Stöber, A. Fink, E. Bohn, Controlled growth of monodisperse silica spheres in the micron size range, J. Colloid Interface Sci. 26 (1968) 62-69. |
| [21] | P. Horcajada, A. Ramila, J. Perez-Pariente, M. Vallet-Regi, Influence of pore size of MCM-41 matrices on drug delivery rate, Microporous Mesoporous Mater. 68 (2004) 105-109. |
| [22] | M. Kruk, M. Jaroniec, A. Sayari, Adsorption study of surface and structural properties of MCM-41 materials of different pore sizes, J. Phys. Chem. B 101 (1997) 583-589. |
| [23] | A.J. Wang, T. Kabe, Fine-tuning of pore size of MCM-41 by adjusting the initial pH of the synthesis mixture, Chem. Commun. (1999) 2067-2068. |
| [24] | L. Coppola, R. Gianferri, I. Nicotera, C. Oliviero, G.A. Ranieri, Structural changes in CTAB/H2O mixtures using a rheological approach, Phys. Chem. Chem. Phys. 6 (2004) 2364-2372. |
| [25] | N.C. Das, H. Cao, H. Kaiser, et al., Shape and size of highly concentrated micelles in CTAB/NaSal solutions by small angle neutron scattering (SANS), Langmuir 28 (2012) 11962-11968. |
| [26] | T. Bellini, N.A. Clark, V. Degiorgio, F. Mantegazza, G. Natale, Light-scattering measurement of the nematic correlation length in a liquid crystal with quenched disorder, Phys. Rev. E 57 (1998) 2996-3006. |
| [27] | H.J. Yun, H. Lee, J.B. Joo, W. Kim, J. Yi, Influence of aspect ratio of TiO2 nanorods on the photocatalytic decomposition of formic acid, J. Phys. Chem. C 113 (2009) 3050-3055. |





