b Laboratory of Clean Energy Chemistry and Materials, Lanzhou Institute of Chemical Physics, Chinese Academy of Science, Lanzhou 730000, China
1. Introduction
Supercapacitors,as energy storage and conversion devices with high power density and fast recharge capability,have promising applications as backup or auxiliary power sources in electric vehicles and other electronic devices [1, 2]. On the basis of the charge storage mechanisms,supercapacitors can be classified into two types: One is the electrical double-layer capacitors (EDLCs), where the capacitance arises from the charge separation at an electrode/electrolyte interface; the other is the pseudo-capacitor, where the capacitance arises from the fast and reversible redox reactions at the surfaces of the electroactive materials [3, 4, 5, 6].
Up to now,various carbon materials,such as activated carbon [7],carbon fibers [8],carbon aerogels [9],carbon nanotubes [10], and grapheme [11],have been used as the electrode materials for EDLCs. Also,some metal oxides/hydroxides (RuO2 [12],MnO2 [13], Co3O4 [14],NiO [15],Co(OH)2 [16],Ni(OH)2 [17],etc.) and conducting polymers (polyaniline [18] and polypyrrole [19]) have been extensively studied as electro-active materials for pseudocapacitors.
Metal organic frameworks (MOFs),a class of crystalline and highly porous hybrid materials obtained by the assembly of metallic ions and organic ligands,have attracted intense interest owing to their wide potential applications in catalysis,magnetism, luminescence,sensors,drug delivery,and particularly in gas storage and separation. These properties and utilities of MOFs depend on their pore size and shape,the interior and exterior surfaces,and the functional groups [20, 21, 22].
More recently,MOFs have been successfully used as electrode materials for rechargeable batteries and fuel cells because the redox behavior of metal cations inside MOFs provides a pathway for electrons,and the linker structure promotes charge transfer inside the framework [23, 24]. The introduction of MOFs in supercapacitors has been reported only recently. Díaz et al. reported for the first time the electrical double-layer capacitive behavior of a Zn-based MOF partially substituted with Co; a gravimetric capacitance of only 2 F g-1 was estimated [25]. Lee et al. synthesized a Co-based MOF and investigated its capacitive behavior in various aqueous electrolytes. The result of the study showed that the Co-based MOF show an interesting pseudocapacitive behavior in LiOH electrolyte [26]. Thus,Morozan et al. deduced that MOFs with abundant porous structure,high surface areas,and incorporated pseudo-capacitive redox centers would be possible candidates as electrode materials for supercapacitors [27]. However,no more convincing experimental instances have been reported to identify such a MOF.
In this work,Ni(II) chloride hydrate and H3btc has been employed for the first time to synthesize Ni-based MOFs materials. The hydrothermal reaction of Ni(II) nitrate hydrate with H3btc acid yields green crystals formulated as Ni3(btc)2·12H2O with a structure composed of zigzag chains with both the bridging and terminal Ni2+ ions and each nickel center coordinated to four water molecules. The electrochemical properties of the prepared Nibased MOF are explored. The test results show that the nickelbased MOF exhibits superior pseudo-capacitive behavior in KOH aqueous electrolyte with a high specific capacitance of 726 F g-1 and good cyclical stability.
2. Experimental 2.1. Synthesis of nickel-based MOF
All solvents and reagents for the syntheses were of analytical grade and were used as received from commercial sources without further purification. A mixture of Ni(Cl)2·6H2O (3.0 mmol),H3btc (1.0 mmol),DMF (N,N-dimethylformamide) (5.0 mL) and distilled water (5.0 mL) was placed in a 25 mL Teflon-lined stainless autoclave. The autoclave was sealed,heated to 105 ℃,kept at 105 ℃ under autogenous pressure for 2 days,and then cooled to room temperature. The as-obtained green crystals were filtered, washed with DMF and distilled water,and then air-dried to give about 0.29 g of Ni-based MOF (yield of 71% based on H3btc). Anal. Calcd. for C18H30Ni3O24 (Mr = 806.5): C 26.81,H 3.75; found: C 26.43,H 3.54.
2.2. Stuctural characterization
A single crystal was selected and attached to a glass fiber using epoxy. Crystal data were collected on a Bruker SMART APEX II diffractomenter at room temperature with graphite-monochromated Mo Ka radiation (l = 0.71073A). The structures were analyzed by direct methods using the SHELXS program.
2.3. Electrochemical measurements in three-electrode system and two electrode system
The electrochemical measurements were carried out using an electrochemical working station (CHI660D,Shanghai,China) in 2 mol L-1 KOH electrolyte at room temperature. The cyclic voltammetry (CV) measurements were conducted at different scan rates ranging from 2.5 mV s-1 to 20 mV s-1. Electrochemical impedance spectroscopy (EIS) measurements were recorded from 10 kHz to 0.1 Hz with an alternate current amplitude of 5 mV. Galvanostatic charge/discharge measurements were run at different current densities ranging from 0.25 A g-1 to 5 A g-1.
For the three-electrode system,the working electrode contained about 8 mg of electroactive material. A platinum gauze electrode and a saturated calomel electrode (SCE) served as the counter electrode and the reference electrode,respectively. The corresponding specific capacitance was calculated from:
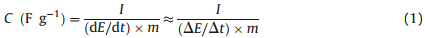
For two-electrode system,the Ni-based MOF cathode and commercial capacitive activated carbon (AC,purchased from Kuraray Co.,Ltd.,Japan) anode electrode were pressed together and separated by a porous nonwoven cloth separator. The electrode contained about 4 mg and 8 mg (according to the equation: C' = IΔt/m,the charge on the MOF and AC electrode are 291 and 152 C -1,respectively. Therefore,the optimal mass ratio of the MOF and AC electrodes in the asymmetric supercapacitor cell is 152:291) of electroactive material for the cathode and anode electrode,respectively. Moreover,a symmetric EDLC (AC/AC) was fabricated for comparison,and each AC electrode contained about 6 mg of electroactive material.
The specific capacitance of the supercapacitor cell can be evaluated from the charge/discharge test together with the following equation:

The specific energy density and power density are defined as

3. Results and discussion
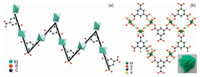
A complete single crystal analysis was undertaken on the asprepared Ni-based MOF,and the crystallographic details of the Nibased MOF were found to have identical cell parameters and single-crystal structure to that of a previously reported cobaltbased MOF [28]. The structural representation of the Ni-based MOF compound is shown in Fig. 1. As shown in Fig. 1(a),the structure of the as-prepared compound is composed of 1-dimentional (1D) zigzag chains constructed from two symmetry-inequivalent tetraaqua nickel (II) units and BTC ligands. As shown in Fig. 1(b),these 1D chains can be reinforced and crosslinked into a complicated 3D network by extensive hydrogen bonding interactions,where every water proton is involved in a hydrogen bond to either a carboxylate or water oxygen.
|
Download:
|
| Fig. 1.(a) The Ni-based MOF chain,presents as the building block in crystalline Ni3(btc)2·12H2O; (b) the 2D sheet motif formed by interchain hydrogen bonds. Hydrogen bonding contacts are shown by the striped bonds. On the lower right corner,a photograph of as-prepared Ni-based MOF is shown. | |
The electrochemical properties of the as-made MOF were investigated under a three-electrode system in KOH aqueous electrolyte. Fig. 2(a) shows the cyclic voltammetry (CV) curves of the Ni-based MOF electrode at different sweep rates. The shape of the CV curves reveals that the capacitance characteristic is well distinguished from that of the EDLCs in which the shape is normally close to an ideal rectangular shape [29]. A couple of redox peaks are observed within the potential range from 0 to 0.5 V. Two plausible reactions may occur as quasi-reversible redox processes during the potential sweep:

|
Download:
|
| Fig. 2.Electrochemical properties of the Ni-based MOF electrode: (a) CV curves at different scan rates; (b) the charge-discharge curves at different current densities; (c and d)Bode plots (the changes absolute impedance and phase angle as a function of frequency). | |

Fig. 2(b) shows the galvanostatic charge/discharge curves of the Ni-based MOF electrode within a potential window of 0-0.4 V at different current densities. The shape of the discharge curves does not display the characteristics of a pure EDLC,but mainly pseudocapacitance, which is in agreement with the result from the CV curves. The corresponding specific capacitance of theMOF electrode at 1 A g-11 was calculated to be 726 F g-11. As the discharge current density increases from 1 A g-11 to 5 A g-11,a large voltage drop is observed,and finally the capacitance decreases from 726 F g-11 to 313.8 F g-11. The decrease in capacitance is attributed to the presence of inner active sites that are unable to sustain the redox transitions completely at higher current densities. This phenomenon may be explained by referring to the OH-1 ion diffusion during the charging/ discharging processes for the electrode. At a relatively high sweep rate with high current density,significant OH-1 ions are required to intercalate swiftly at the interface of electrode/electrolyte,however, the unfavorable accessibility of the ions and the relatively low concentration of OH-1 ions cannot meet this demand [31].
To evaluate the conductivity and charge transport properties at the electrode/electrolyte interface,electrical impedance spectroscopy (EIS) was performed in a frequency range from 0.1 Hz to 10 kHz. The changes in absolute impedance and phase angle as a function of frequency are shown in Fig. 2(c) and (d). As shown in Fig. 2(c),the total impedance of the electrode increases with the decrease of the frequency. Moreover,as shown in Fig. 2(d),the Bode plot of the electrode has a small peak at a frequency of 103 Hz, indicating that the electrode has a smaller charge-transfer resistance. In addition,the value of the phase angle only reaches 57° (<90°) for the electrode when the frequency increases to 10 Hz, which demonstrates that the electrode is mainly controlled by ion diffusion process [32].
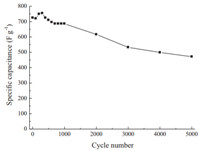
The cycle life of the resultant Ni-based MOF electrode was monitored by a chronopotentiometry measurement at 1 A g-11 in 2 mol L-11 KOH electrolyte. As shown in Fig. 3,we can find that in the first 1000 cycles the value of the specific capacitance can keep 94.5% of the initial value,and even as the electrode cycles 2000 times it still remains at 85% initial value,indicating good cycling stability. The main recession of the capacitance occurs between 2000 and 3000 cycles,and after a continuous 5000 cycles,the value of the specific capacitance remains 65% of the initial value. So,the main cause of the decline of the capacitance may be attributed to that fact that during the continuous cycles,the OH-1 ions repeatedly intercalate and deintercalate at the interface of electrode/electrolyte,which can lead to the structure collapse of Ni-MOFs and then hinder the electrolyte wetting electrode.
|
Download:
|
| Fig. 3.Cycle life of the Ni-based MOF electrode at the current density of 1 A g-11 in KOH electrolyte. | |
Considering the high pseudo-capacitance character of MOF materials and the fast ion-transport property of the EDLC storage for activated carbon (AC) materials,a simple asymmetric supercapacitor was fabricated using the as-made MOF material as the positive electrode and AC as the negative electrode,respectively. Fig. 4(a) shows the CV curves of the MOF/AC asymmetric supercapacitor at different voltage scan rates between 0.4 and 1.6 V in 2 mol L-11 KOH aqueous electrolyte. Apart from the increase of current,the CV profiles still retain the original shapes with increasing potential scan rates,indicating the desirable CV reversibility at an enlarged potential window of 1.2 V and the fast charge/discharge property of the fabricated asymmetric supercapacitor.

|
Download:
|
| Fig. 4.Electrochemical properties of the MOF/AC asymmetric supercapacitor in 2 mol L-11 KOH electrolyte within a cell voltage from 0.4 V to 1.6 V: (a) CV curves at different scan rates; (b) chare/discharge curves at different current densities. The comparison between the MOF/AC asymmetric supercapacitor and AC/AC symmetric supercapacitor: (c) specific capacitance as a function of discharge current density and (d) ragone plot relating power density to achievable energy density. | |
Fig. 4(b) shows the galvanostatic charge-discharge curves of the hybrid cell between 0.4 and 1.6 V at different current densities. It can be observed that the shape of all the charge-discharge curves for the asymmetric supercapacitor is closely linear and shows a typical triangle symmetrical distribution,displaying a good capacitive property.
In comparison,a simple symmetric supercapacitor was also fabricated using the AC material as the negative and positive electrodes. Fig. 4(c) shows the specific capacitance as a function of the discharge current density for the Ni-based MOF/AC asymmetric and AC/AC symmetric supercapacitors. As the values show, within the whole range of current densities,the specific capacitance of the MOF/AC asymmetric supercapacitor is much higher than that of the AC/AC symmetric supercapacitor. In addition,the power and energy density of the two supercapacitors were evaluated and the corresponding values are shown in Fig. 4(d). It is clear that the MOF/AC asymmetric supercapacitor has a relatively high specific energy density (16.5 Wh kg-11) and power density (2078 Wh kg-11). Its specific energy density is quadrupled compared with that of the AC/AC symmetric supercapacitor (3.98 Wh kg-11).
4. Conclusion
In summary,through studying the electrochemical properties of the Ni3(btc)2·12H2O MOF material,a new application of the nickel-based MOF as a pseudo-capacitive material for supercapacitors is investigated. It exhibits superior pseudo-capacitive behavior with high specific capacitance and good electrochemical stability. Predictably,the current identification probably provides a new direction to explore the energy storage behavior and mechanism of more MOFs,and gives a new insight for designing and synthesizing MOFs-based electrode materials for highperformance supercapacitors and other energy-storage devices.
Acknowledgment
This work was supported by the National Natural Science Foundation of China (No. 21203223).
| [1] | M. Winter, R.J. Brodd, What are batteries, fuel cells, and supercapacitors? Chem. Rev. 104 (2004) 4245-4269. |
| [2] | P. Simon, Y. Gogotsi, Materials for electrochemical capacitors, Nat[42_TD$DIF]. Mater. 7 (2008) 845-854. |
| [3] | S.H. Aboutalebi, A.T. Chidembo, M. Salari, K. Konstantinov, D. Wexler, Comparison of GO, GO/MWCNTs composite and MWCNTs as potential electrode materials for supercapacitors, Energy Environ. Sci. 4 (2011) 1855-1865. |
| [4] | Z.S. Wu, D.W. Wang, W.C. Ren, et al., Anchoring Hydrous RuO2 on grapheme sheets for high-performance electrochemical capacitors, Adv. Funct. Mater. 20 (2010) 3595-3602. |
| [5] | J.W. Lang, L.B. Kong, W.J. Wu, Y.C. Luo, L. Kang, Facile approach to prepare loosepacked NiO nano-flakes materials for supercapacitors, Chem. Commun. 35 (2008) 4213-4215. |
| [6] | Y.W. Zhu, S. Murali, M.D. Stoller, et al., Carbon-based supercapacitors produced by activation of graphene, Science 332 (2011) 1537-1541. |
| [7] | M.X. Liu, L.H. Gan, W. Xiong, et al., Partially graphitic micro-and mesoporous carbon microspheres for supercapacitors, Chin. Chem. Lett. 24 (2013) 1037-1040. |
| [8] | X.B. Yan, Z.X. Tai, J.T. Chen, Q.J. Xue, Fabrication of carbon nanofiber-polyaniline composite flexible paper for supercapacitor, Nanoscale 3 (2011) 212-216. |
| [9] | C. Moreno-Castilla, M.B. Dawidziuk, F. Carrasco-Marín, Z. Zapata-Benabithe, Surface characteristics and electrochemical capacitances of carbon aerogels obtained from resorcinol and pyrocatechol using boric and oxalic acids as polymerization catalysts, Carbon 49 (2011) 3808-3819. |
| [10] | X. Zhao, B.T.T. Chu, B. Ballesteros, et al., Spray deposition of steam treated and functionalized single-walled and multi-walled carbon nanotube films for supercapacitors, Nanotechnology 20 (2009) 065605. |
| [11] | W.W. Liu, X.B. Yan, J.W. Lang, Q.J. Xue, Electrochemical behavior of graphene nanosheets in alkylimidazolium tetrafluoroborate ionic liquid electrolytes: influences of organic solvents and the alkyl chains, J. Mater. Chem. 21 (2011) 13205-13212. |
| [12] | T.P. Gujar, V.R. Shinde, C.D. Lokhande, W.Y. Kim, Spray deposited amorphous RuO2 for an effective use in electrochemical supercapacitor, Electrochem. Commun. 9 (2007) 504-510. |
| [13] | M.W. Xu, L.B. Kong, W.J. Zhou, H.L. Li, Hydrothermal synthesis and pseudocapacitance properties of α-MnO2 hollow spheres and hollow urchins, J. Phys. Chem. C 111 (2007) 19141-19147. |
| [14] | J.W. Lang, X.B. Yan, Q.J. Xue, Facile preparation and electrochemical characterization of cobalt oxide/multi-walled carbon nanotube composites for supercapacitors, J. Power Sources 196 (2011) 7841-7846. |
| [15] | A.I. Inamdar, Y.S. Kim, S.M. Pawar, et al., Chemically grown, porous, nickel oxide thin-film for electrochemical supercapacitors, [43_ TD$D IFF]J. Power Sources 196 (2011) 2393-2397. |
| [16] | W.J. Zhou, M.W. Xu, D.D. Zhao, C.L. Xu, H.L. Li, Electrodeposition and characterization of ordered mesoporous cobalt hydroxide films on different substrates for supercapacitors, Microporous Mesoporous Mater. 117 (2009) 55-60. |
| [17] | G.W. Yang, C.L. Xu, H.L. Li, Electrodeposited nickel hydroxide on nickel foam with ultrahigh capacitance, Chem. Commun. (2008) 6537-6539. |
| [18] | J.J. Cai, L.B. Kong, J. Zhang, Y.C. Luo, L. Kang, A novel polyaniline/mesoporous carbon nano-composite electrode for asymmetric supercapacitor, Chin. Chem. Lett. 21 (2010) 1509-1512. |
| [19] | D.P. Dubal, S.H. Lee, J.G. Kim, W.B. Kim, C.D. Lokhande, Porous polypyrrole clusters prepared by electropolymerization for a high performance supercapacitor, J. Mater. Chem. 12 (2012) 3044-3052. |
| [20] | N. Stock, S. Biswas, Synthesis of metal-organic frameworks (MOFs): Routes to various MOF topologies, morphologies, and composites, [45_TD$DIF]Chem. Rev. 112 (2012) 933-969. |
| [21] | S.Q. Su, W. Chen, X.Z. Song, et al., Three unprecedented open frameworks based on a pyridyl-carboxylate: synthesis, structures and properties, [46_TD$DIF]CrystEngComm 14 (2012) 1681-1686. |
| [22] | H.H. Wu, Q.H. Gong, D.H. Olson, J. Li, Commensurate adsorption of hydrocarbons and alcohols in microporous metal organic frameworks, Chem. Rev. 112 (2012) 836-868. |
| [23] | Y. Kobayashi, B. Jacobs, M.D. Allendorf, J.R. Long, Conductivity, doping, and redox chemistry of a microporous dithioleneobased metal-organic framework, Chem. Mater. 22 (2010) 4120-4122. |
| [24] | J.N. Behera, D.M. D'Alessandro, N. Soheilnia, J.R. Long, Synthesis and characterization of ruthenium and iron-ruthenium Prussian blue analogues, Chem. Mater. 21 (2009) 1922-1926. |
| [25] | R. Díaz, M.G. Orcajo, J.A. Botas, G. Calleja, J. Palma, Co8-MOF-5 as electrode for supercapacitors, Mater. Lett. 68 (2012) 126-128. |
| [26] | D.Y. Lee, S.J. Yoon, N.K. Shrestha, et al., Unusual energy storage and charge retention on Co-based metal-organic-frameworks, Microporous Mesoporous Mater. 153 (2012) 163-165. |
| [27] | A. Morozan, F. Jaouen, Metal organic frameworks for electrochemical applications, Energy Environ. Sci. 5 (2012) 9269-9290. |
| [28] | O.M. Yaghi, H.L. Li, T.L. Groy, Construction of porous solids from hydrogen-bonded metal complexes of [48_TD$DIF][49_DIF]1,3,5-benzenetricarboxylic acid, J. Am. Chem. Soc. 118 (1996) 9096-9101. |
| [29] | M.F. El-Kady, V. Strong, S. Dubin, R.B. Kaner, Laser scribing of high-performance and flexible graphene-based electrochemical capacitors, Science 335 (2012) 1326-1330. |
| [30] | X. Sun, G.K. Wang, J.Y. Hwang, J. Lian, Porous nickel oxide nano-sheets for high performance pseudocapacitance materials, J. Mater. Chem. 21 (2011) 16581-16588. |
| [31] | R. Kötz, M. Carlen, Principles and applications of electrochemical capacitors, Electrochim. Acta 45 (2000) 2483-2498. |
| [32] | W.W. Liu, X.B. Yan, J.W. Lang, J.T. Chen, Q.J. Xue, Influences of the thickness of selfassembled grapheme multilayer films on the supercapacitive performance, Electrochim. Acta 60 (2012) 41-49. |




