1. Introduction
In recent years, the increasing water pollution has caused potentially harmful micrograms and organic chemicals to manifest and grow to extremely dangerous levels [1]. The widespread use of antibiotics made the microbial organisms, such as harmful anaerobic and aerobic bacteria, more resistant to its exposure. Moreover, some antimicrobial agents are extremely irritant and toxic, and there is a great deal of interest in finding ways to formulate new varieties of safe and cost-effective bactericidal materials [2]. Chemical disinfectants commonly used for wastewater treatment such as detergents, alcohol, chlorine components, etc., reacts with the natural water sources to form DBPs or disinfection by-products, many of which are carcinogens. More than 600 DBPs have been reported in literature so far [3,4]. Microbial contamination imparts threat to human health, and is thus necessary to be removed. UV irradiation has proven to be effective but temporary solution to the problem. Thus photocatalysis science has emerged to be a novel, cost effective and simpler method for water purification, purifying water sources from organic chemicals and microbes. So far, various semiconductor materials such as TiO2, ZnO, CdS, etc., have been previously investigated for their photocatalytic activity. However, the materials possess high band gap energies which can be a significant disadvantage. The band gap energies of TiO2, ZnO, and CdS are 3.0 eV, 3.4 eV and 2.4 eV respectively [5–10]. Among the promising carbon materials, graphene, a flat monolayer of carbon atoms tightly packed into a two-dimensional (2D) honeycomb lattice structure, is expected to have great potential as a nanoscale building block for developing hybrid materials due to its unique sheet morphology, ultrahigh electron conductivity, and mobility [11]. In this paper, the photocatalytic behavior of CdSe particles after being attached on graphene was investigated when irradiated with visible light and their potential an antimicrobial agent was also evaluated. S. aureus was used for the investigation of the possible bactericidal effect of the CdSegraphene composites.
2. Experimental 2.1. Materials
Graphene oxide was prepared in the laboratory from pristine graphite following the Hummer’s-Offeman method as done in several of our previous experiments [12]. The prepared graphene oxide were used in the formation of the CdSe-graphene composites. Cadmium acetate [Cd(CH3COO)2, 98%], selenium powder (Se, 99%), ammonium hydroxide (NH4OH, 25–28%), were purchased from Daejung Chemicals Co., Ltd., Korea. Whereas anhydrous sodium sulfite (Na2SO3, 95%) was purchased from Duksan pharmaceutical Co., Ltd., Korea. Methylene blue trihydrate (C16H18CIN3S·3H2O, 98%) was purchased from Samchun pure chemicals Co., Ltd., Korea. All the chemicals were used without further purification and all experiments were carried out using distilled water.
2.2. Synthesis of CdSe precursors
CdSe precursor materials were synthesized using 4 g of anhydrous sodium sulfite (Na2SeSO3) and 0.2 g of crude selenium powder (Se). In a reaction vessel these chemicals were vigorously stirred with 20 mL of distilled water, maintaining a constant temperature of 70 8C to form Na2SeSO3 solution. In another reaction vessel, 2 mL of ammonium hydroxide solution (NH4OH) was added to an aqueous solution of 0.675 g of cadmium acetate [Cd(CH3COO)2] to form Cd (NH3)4 2+ solution. After the formation of Cd (NH3)4 2+ solution, it was quickly added to Na2SeSO3 solution with continued stirring for 3 h at 80 8C. The solution was allowed to cool at room temperature and the CdSe precipitates were obtained. The precipitates were filtered using 47 mm Whatman filter paper with 0.7 μm pore size. The residue was washed with distilled water at least 5 times. The CdSe powder was collected and dried in vacuum oven at a temperature of 353 K for 8 h.
2.3. Synthesis of CdSe-graphene composites
CdSe-graphene composite particles were prepared relatively with a simple hydrothermal process. In this process, the previously obtained CdSe particles were taken in 200 mL distilled water to which 1 g of graphene oxide (previously obtained by Hummer’s- Offeman method) was added in a ratio of 1:1. After a hydrothermal reaction at 70 8C at the end of 6 h, graphene oxide was reduced to graphene nanosheet and CdSe compounds naturally grew on its surface to generate a graphene-CdSe composite. After the stirring is stopped, the resultant is allowed to cool, settle at room temperature and filtered with 47 mmwhatman filter paper having a pore size of 0.7 mm. The resultant powder was washed with distilled water 3 times and finally it was dried in vacuum oven. The powder was heated to 100 8C for 8 h in vacuum oven to form the CdSe-graphene composites.
2.4. Reusibility test
The reusibility test of CdSe-graphene composites was performed with cleaning the composites used previously for the photocatlytic degradation of MB. Firstly, the composites were immersed in ethanol for 6 h and rinsed with deionized water. Then the composites were dried in hot air oven at a constant temperature of 353 K. After the drying process, the cleaned CdSe-graphene composites were reused for removing dyes, and the cyclic experiment was repeated several times.
2.5. Characterization
To identify the crystalinity of the preapared compound, XRD (Shimadzu XD-D1) with monochromatic high intensity CuKa radiation (λ = 1.5406A) was done. SEM (JSM-5600 JEOL, Japan) was used to observe the surface structure of the prepared composites. Additionally, EDX analysis (attached to SEM) was done in order to perform the elemental analysis of the desired region of the prepared CdSe and CdSe-graphene composites. A more detailed study of the composite particles was possible with TEM (JEOL, JEM-2010, Japan). The specific surface area (BET) was determined by N2 adsorption measurements at 77 K (MONOSORB, USA). X-ray photoelectron spectroscopy (XPS) was performed using VG scientific VISACA lab 2000, with a monochromatic Mg Xray radiation source. Survey (wide scan) spectra and highresolution spectra of C 1 s, and the element contained within sample, were recorded. The change in absorbance was measured using UV–vis spectrophotometer (Optizen POP, Korea).
2.6. Bacterial strain and growth conditions
S. aureus was obtained from the American Type Culture Collection (ATCC). The strain was grown in Luria–Bertani (LB) medium at 37 8C. All inoculation experiments used were kept in an overnight accumulation culture grown to stationary phase in advance. The initial culture absorbance A600 was 0.04. Bacterial growth was assessed by using the time dependent absorbance curve. The cell concentration was estimated by the turbidityspectra method [13].
2.7. Analysis of photocatalytic effect
In order to analyze the photocatalytic effect of CdSe-graphene composites, the decomposition reaction of MB in aqeuous solution was performed. Prior to illumination, 0.3 g of powdered sample were dispersed in 100 mL of (1 × 10-4 mol/L) aqueous MB solution using magnetic stirrer under dark ambiance for 30 min. The first 30-min interval were necessary to achieve adsorption/desorption equilibrium. For irradiation, a LED lamp (8 W, Fawoo, Lumidas-H) was used at a distance of 90 mm from the solution in the dark box. The suspension was irradiated with visible light as a function of irradiation time. The samples were withdrawn regularly from the reactor at regular intervals of 0 min, 30 min, 60 min, 90 min, 120 min, and 150 min respectively and the dispersed powders were removed using a centrifuge. The clean transparent solution was analyzed by UV–vis spectroscopy.
The percentage degradation of MB was obtained by the following formula:
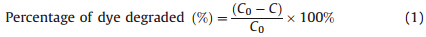
2.8. Measurement of antibacterial performance
Halo test: The test was initiated by pouring the nutrient agar onto sterilized Petri dishes and was allowed to solidify, and then 50 μL of incubated testing bacterial solution (106 CFU/mL) was spread uniformly over the plate. 2–10% solutions were inoculated on the surface of agar with S. aureus. The samples with and without anti-bacterial agent were gently dropped over solidified agar gel in the same Petri dishes which had duplication for obtaining the average. The Petri dishes were then incubated at 37 8C and examined after 24 h for a zone of inhibition. The diameter of inhibition zone was then measured. After incubation, the diameter of the zone of bacterial-growth inhibition was measured with an accuracy of ±0.1 mm. The mean inhibition-zone diameter and the maximal data scatter also were determined. All experiments were repeated thrice.
3. Results and discussion
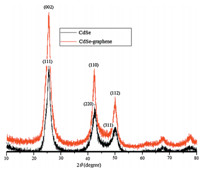
The X-ray diffraction (XRD) patterns of CdSe and CdSegraphene
composites are shown in Fig. 1 and 2. For CdSe
compound, the XRD diffraction peaks around 2θ of 25.4°, 42°, and
49.7° which can be indexed to the characteristic peaks (1 1 1),
(2 2 0), and (3 1 1) plane reflections of cubic crystal structure CdSe
with lattice constants of 6.05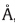 , according to standard power
diffraction data (JCPDS No. 65-2891 for CdSe, cubic) [14]. However,
for CdSe-graphene nanocomposites only peaks of CdSe were
detected. The (0 0 2) and (1 1 0) reflections around 2θ of 25.3°, and
41.8° of CdSe respectively were detected in CdSe-graphene
nanocomposites because graphene peaks were overlaped by the
cubic (1 1 1) and (2 2 0) reflections around 2θ of 25.4° and 48° of
CdSe. Therefore, the intensity peaks at 25.4° and 42° in CdSe
composite was stronger than CdSe-graphene and the micromorphology
of CdSe is different than the mixture of graphene and CdSe.
, according to standard power
diffraction data (JCPDS No. 65-2891 for CdSe, cubic) [14]. However,
for CdSe-graphene nanocomposites only peaks of CdSe were
detected. The (0 0 2) and (1 1 0) reflections around 2θ of 25.3°, and
41.8° of CdSe respectively were detected in CdSe-graphene
nanocomposites because graphene peaks were overlaped by the
cubic (1 1 1) and (2 2 0) reflections around 2θ of 25.4° and 48° of
CdSe. Therefore, the intensity peaks at 25.4° and 42° in CdSe
composite was stronger than CdSe-graphene and the micromorphology
of CdSe is different than the mixture of graphene and CdSe.
The specific surface area (BET) of graphene and CdSe-graphene were determined. BET surface area of graphene was found to be 43.5 m2/g and for CdSe-graphene it was 12.5 m2/g. From the values it is evident that the graphene nanosheets have greater BET value than CdSe-graphene which can effect the adsorption reaction time. The decrease in surface area may be due to agglomeration of graphene oxide sheet during hydrothermal treatment. Another reason for the reduced surface area may be the agglomerated CdSe particles on the surface of graphene oxide sheet which is evident from the dark regions on TEM images. The surface characteristics of CdSe-graphene composites were determined by SEM micrographs. The SEM micrographs of CdSe-graphene is shown in Fig. S1 in Supporting information. From Fig. S1(a and b), very uniform sperical shaped CdSe particles were observed that agglomerated together. For CdSe-graphene composites, the sperical shaped agglomerated CdSe particles were observed attached to the surface of the graphene nanosheets and the distribution of the CdSe particles with the graphene nanosheets were found to be uneven. More detailed information of the surface state can be confirmed by the TEM. As shown in the TEM image of CdSe-graphene in Fig. 2(a and b) the CdSe particles were decorated on the surface of graphene nanosheets. In CdSe-graphene nanocomposites, the graphene sheet was decorated with CdSe nanoparticles having size of ~10 nm.
|
Download:
|
| Fig. 1.XRD patterns of CdSe compound, CdSe-graphene composites. | |

|
Download:
|
| Fig. 2.TEM images of CdSe-graphene composite. | |
XPS was used for qualitative analysis of composite containing CdSe-graphene nanocomposites. The survey spectrum had peaks corresponding to Cd, O, C and Se consistent with the formation of CdSe-graphene composite as shown in Fig. S2(a) in Supporting information. From Fig. S2(b) two main peaks of Cd 3d core level corresponds 3d3/2 at 404.9 eV and 3d5/2 at 412.1 eV were observed. The peak corresponding to O 1s was at 532.75 eV can be seen in Fig. S2(c) [23]. The C 1s region had a strong peak located at 284.01 eV as depicted in Fig. S2(d), which corresponded to the C=C group. Furthermore C 1s spectra exhibited weak peaks at 287.1 eV, 289.01 eV corresponding to C–O, C=O groups. These results indicate that our composite consist of slight reduced graphene oxide sheet. Selenium 3d core level peak were confirmed at 54.60 eV form the high resolution scan spectra as shown in Fig. S2(e) [24]. The photocatalytic degradation of the prepared sample was evaluated under visible light. Fig. S3 in Supporting information shows the time series of dye degradation using CdSe-graphene on MB. The spectra of the dye solution show the relative degradation yields at different time intervals. The decreasing concentration of MB in the photocatalytic reaction, was used to evaluate the activity of CdSe graphene composites. Fig. 3 represents the degradation of MB with CdSe-graphene under visible light from which it was clear that the concentration of MB gradually diminished with increasing time. Moreover, the dye solution increasingly lost its color intensity as the dye concentration continued to decrease. The decrease in concentration was evaluated at the λmax value of the dye which were determined from the absorption spectra. The lmax value of MB was found to be 665 nm. In case of CdSe, MB was degraded to an extent of 75.2% at the end of 150 min, shown in Fig. 3. However, toward the end of 150 min there was a little attainment of saturation point which may be accounted to the dye molecules being in constant contact with composites preventing the new dye molecules from coming in contact with the composite surface. Also with the withdrawal of each sample over the period caused a decrease in the amount of composites in the reaction vessel which may have led to the saturation factor. MB was photodegraded by cleavage and demethyletion that caused the decoloration of the dye solution. According to previous reports [15], MB suspended in photocatalyst irradiated with visible light reacts with photogenerated electron (eCB-1) to produce leuco form (LMB) under anaerobic condition Eq. (2), while MB oxidizes under aerobic condition Eq. (3).

|
Download:
|
| Fig. 3.The decrease in concentration of MB exposure to CdSe-graphene photocatalyst under visible light at various time intervals. | |
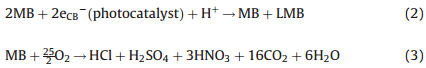
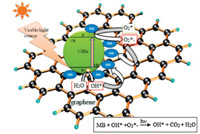
The scheme of charge transfer process between CdSe and graphene sheet is shown in Fig. 4. When CdSe-graphene was illuminated with visible light the semiconductor (metal selenide) produced electrons (e-1) and holes (h+) that gained enough energy or momentum to overcome the energy barrier denoted as Eg. The electrons (e-1) travels from the valence band (VB) to the conduction band (CB), producing increased number of electrons (e-1) in the conduction band (CB) and holes (h+) in the valence band (VB). Thus, a number of electrons (e-1) and holes (h+) were generated in CdSe. Meanwhile, graphene attached to the semiconductor material (CdSe) assisted in the smooth transistion transferring electrons (e-1) to the CB of CdSe, thereby increasing the number of electrons as well as the rate of electron-induced redox reactions. The graphene coupled CdSe system had enhanced photocatalytic activity mainly because of the high charge seperation induced by the synergistic effects of graphene on CdSe. The generated electrons (e-1) react with dissolved oxygen molecules and produce oxygen peroxide radicals O2·-1. The positive charge hole (h+) can react with OH-1 derived from H2O to form hydroxyl radicals OH·. The MB molecules then can be photocataytically degraded by oxygen peroxide radicals O2·-1 and hydroxyl radicals OH· to CO2, H2O, and other mineralization products. The reactions involved in the charge mobility and mineralization of the dyes are as follows:
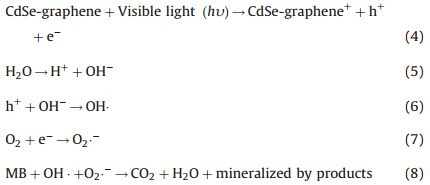
|
Download:
|
| Fig. 4.The schematic representation of the photocatalytic degradation mechanism of MB by CdSe-graphene in presence of visible light. | |


|
Download:
|
| Fig. 5.Apparent first-order reaction kinetics of photocatalytic degradation of MB with CdSe-graphene. | |

The antibacterial activity of CdSe-graphene against S.aureus was investigated due to its easy availability for its ubiquitous presence. For the purpose of comparison, we used antibiotic kanamycin as standard, given in the culture media of the bacteria. The bacterium S.aureus was treated with kanamycin as standard and then was treated with three different concentrations, i.e., 2%, 5% and 10% of CdSe-graphene solutions. Fig. 6(a) showed excellent bactericidal effect of antibiotic kanamycin, restricting the growth of bacterial colonies and forming clear zone of approximate diameter of 2.0 ± 0.3 cm. The transparent clear zone represented the area not being infected by the bacterium. Simultaneously, in order to determine the minimum inhibitory concentration (MIC), the bacterium culture was treated with three different solutions of CdSegraphene composites (2%, 5% and 10%), the composite solution containing 10% of CdSe-graphene showed satisfactory results. A clear inhibition zone of around 1.4 ± 0.1 cm was observed indicating the existence of antibacterial effect of CdSe-graphene composites given at an appropriate concentration. Observing the inhibition zone created by increased concentration of the composites, the concentrations of the solutions were increased further to check its effect on the bacterium culture. As a result, in another bacterium culture three more concentrations of 10%, 15% and 20% solutions of CdSe-graphene composite materials were added along with standard kanamycin. It was observed that inhibition zones of 1.5 ± 0.7 cm, 1.3 ± 0.7 cm and 1.4 ± 0.6 cm were created by 10%, 15% and 20% solutions of CdSegraphene composites as shown in Fig. 6(b). Thus, 10% CdSe-graphene solutions showed optimum results as compared to all the other concentrations. The generally accepted reason for semiconductor composite materials to show significant antibacterial activity against various bacterial species has been identified as the interaction with highly reactive oxygen species (ROS) with the cell membrane of the bacterium which would result in oxidative damage of the cell membrane or inside the cells [21]. The ROS can include hydroxyl groups, superoxide anions (O2·-), and hydrogen peroxide. The photocatalytic activity gives us a subtle indication because it is possible that the photocatalytic reactions cause the damage of the cell membrane and as in previous case studies done with TiO2, ZnO nanorods, etc. [22].

|
Download:
|
| Fig. 6.Photograph of bactericidal effect observed by halo test for bacterium Streptococcus aureus with standard antibiotic Kanamycin and (a) 2%, 5% and 10% of CdSe-graphene composite solutions (b) 10%, 15% and 20% of CdSe-graphene composite solutions. | |
4. Conclusion
Highly pure CdSe-graphene was prepared with simple hydrothermal method. From the XRD patterns of CdSe-graphene composites typical cubic lattice structure of CdSe was observed. From the SEM and TEM results, for CdSe-graphene composites, spherical-shaped agglomerated CdSe particles were observed on the surface of graphene nanosheets. The CdSe particle sizes were around 10 nm, uniformly attached on the graphene surface with other CdSe particles to form small clusters. In the present study, it was found that CdSe-graphene acted as an excellent photocatalyst material. The decrease in absorbance was evident with UV–vis spectroscopic analysis. The decrease in concentration of the dye solutions was observed with decreasing color intensity of the dye with increasing time. At the end of 150 min 75.2% of MB was degraded. On treating the bacterium culture of S.aureus with 2%, 5% and 10% solutions of CdSe-graphene composites, it was observed that 10% solution of CdSe-graphene composites showed satisfactory bactericidal effect. Thus, it can be concluded that CdSegraphene can not only photocatalytically degrade the organic dyes but can also kill the microbes present in the water sources.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.cclet.2014.03.050.
| [1] | N.Talebian, M.R. Nilforoushan, E.B. Zargar, Enhanced antibacterial performance of hybrid semiconductor nanomaterials ZnO/SnO2 nanocomposites thin films, Appl. Surf. Sci. 258 (2011) 547-555. |
| [2] | F.J. Zhang, W.C. Oh, Characterization and photonic effect of novel Ag-CNT/TiO2 composites and their bactericidal activities, Bull. Korean Chem. Soc. 31 (2010) 1981-1987. |
| [3] | S.W. Krasner, H.S. Weinberg, S.D. Richardson, et al., Occurrence of a new generation of disinfection byproduct, Environ. Sci. Technol. 40 (2006) 7175-7185. |
| [4] | S.I. Abou-Elela, H.S. Ibrahim, M.M. Kamel, M. Gouda, Application of nanometal oxides in situ in nonwoven polyester fabric for the removal of bacterial indicators of pollution from wastewater, Sci. World J. 2014 (2014) 950348. |
| [5] | L.B. Reutergardh, M. Iangphasuk, Photocatalytic decolorization of reactive azo dye: a comparison between TiO2 and CdS photocatalysis, Chemosphere 35 (1997) 585-596. |
| [6] | K.Ullah, L. Zhu, Z.D. Meng, et al., A facile and fast synthesis of novel composite Pt-graphene/TiO2 with enhanced photocatalytic activity under UV/visible light, Chem. Eng. J. 231 (2013) 76-83. |
| [7] | K.Moazzami, T.E. Murphy, J.D. Phillips, M.C.K. Cheung, A.N. Cartwright, Subbandgap photoconductivity in ZnO epilayers and extraction of trap density spectra, Semicond. Sci. Technol. 21 (2006) 717-723. |
| [8] | K.Ullah, S. Ye, L. Zhu, et al., Microwave assisted synthesis of a noble metalgraphene hybrid photocatalyst for high decomposition of organic dyes under visible light, Mater. Sci. Eng. B 180 (2014) 20-26. |
| [9] | Z.D. Meng, L. Zhu, S. Ye, et al., Fullerene modification CdSe/TiO2 and modification of photocatalytic activity under visible light, Nanoscale Res. Lett. 8 (2013) 189. |
| [10] | T.Ghosh, K. Ullah, J.H. Lee, et al., Graphene oxide based CdSe photocatalysts: synthesis, characterization and comparative photocatalytic efficiency of rhodamine B and industrial dye, Mater. Res. Bull. 48 (2013) 1268-1274. |
| [11] | K.S. Novoselov, A.K. Geim, S.V. Morozov, et al., Electric field effect in atomically thin carbon films, Science 306 (2004) 666-669. |
| [12] | Z.D. Meng, L. Zhu, K. Ullah, et al., Enhanced visible light photocatalytic activity of Ag2S-graphene/TiO2 nanocomposites made by sonochemical synthesis, Chin. J. Catal. 34 (2013) 1527-1533. |
| [13] | S.Y. Shchyogolev, N.G. Khlebtsov, B.I. Schwartsburd, Spectroturbidimitry as applied to biomedical and immunological investigations, in: Proc. SPIE 1981, Optical Methods of Biomedical Diagnostics and Therapy, SPIE, Saratov, Russia, 1993. |
| [14] | T.Ghosh, K. Ullah, V. Nikam, et al., The characteristic study and sonocatalytic performance of CdSe-graphene as catalyst in the degradation of azo dyes in aqueous solution under dark conditions, Ultrason. Sonochem. 20 (2013) 768-776. |
| [15] | C.Yogi, K. Kojima, N. Wada, et al., Photocatalytic degradation of methylene blue by TiO2 film and Au particles-TiO2 composite film, Thin Solid Films 516 (2008) 5004-5881. |
| [16] | L.L. Zhang, Y.L. Nie, C. Hu, X.X. Hu, Decolorization of methylene blue in layered manganese oxide suspension with H2O2, J. Hazard. Mater. 190 (2011) 780-785. |
| [17] | Y.Li, X. Li, J. Li, J. Yin, Photocatalytic degradation of methyl orange by TiO2 coated activated carbon and kinetic study, Water Res. 40 (2006) 1119-1126. |
| [18] | R.P. Schwarzenbach, P.M. Gschwend, D.M. Imboden, Environmental Organic Chemistry, 2nd ed., John Wiley and Sons, New York, 2002. |
| [19] | T.Ghosh, W.C. Oh, Review on reduced graphene oxide by chemical exfoliation method and its simpler alternative of ultrasonication and heat treatment method for obtaining graphene, J. Photocatal. Sci. 3 (2012) 17-23. |
| [20] | T.Ghosh, K.Y. Cho, K. Ullah, et al., High photonic effect of organic dye degradation by CdSe-graphene-TiO2 particles, J. Ind. Eng. Chem. 19 (2013) 797-805. |
| [21] | S.Makhluf, R. Dror, Y. Nitzan, et al., Microwave-assisted synthesis of nanocrystalline MgO and its use as a bacteriocide, Adv. Funct. Mater. 15 (2005) 1708-1715. |
| [22] | M.S. Wong, W.C. Chu, D.S. Sun, et al., Visible-light-induced bactericidal activity of a nitrogen-doped titanium photocatalyst against human pathogens, Appl. Environ. Microb. 72 (2006) 6111-6116. |
| [23] | S.A. Vanalakar, S.S. Mali, R.C. Pawar, et al., Synthesis of cadmium sulfide spongy balls with nanoconduits for effective light harvesting, Electrochim. Acta 56 (2011) 2762-2768. |
| [24] | A.Benayad, H.J. Shin, H.K. Park, et al., Controlling work function of reduced graphite oxide with Au-ion concentration, Chem. Phys. Lett. 475 (2009) 91-95. |




