1. Introduction
Molecule-based magnetic materials,such as single-molecular magnets (SMMs),photomagnets,magneto-optical materials, magnetic conductors,ferroelectromagnets,chiral magnets,and multifunctional materials have attracted increasing interest over the last few years [1]. The design and synthesis of SMMs is of particular interest,for their magnetic bistability potentially allows devices for ultimate high-density memory storage and quantum computing [2, 3]. Manganese based SMMs have been studied the most,with focus on the paramagnetic nature of the manganese ion in various oxidation states,which provides interesting magnetic properties [4, 5, 6]. Detailed studies of the solid state and frozen solution magnetic properties of a family of oxime-based hexanuclear Mn(III) SMMs (Mn6 SMMs) led to the first established solid state magnetostructural correlations for any SMM,demonstrating that their solid state magnetic properties are crucially dependent on very small changes in geometry; in particular,the Mn-N-O-Mn torsion angles of the metal oxime core [7, 8].
We are interested in introducing other transition metal ions into the Mn6 system and studying how structure change affecting the magnetic properties. In this paper,we report the synthesis, structural characterization,and magnetic properties study of a bimetallic hexanuclear cluster based on 5-chlorosalicylaldehyde oxime (Cl-H2Sao),[Mn4Ni2O2(Cl-Sao)6·(CH3OH)8]·10CH3OH.
2. Experimental
All chemicals and solvents were commercial products and used
without further purification. Elemental analyses of C,H,and N
were performed by a Carlo Erba 1106 elemental analyzer. Metal
analyses of Mn and Ni were performed by ICP-AES. IR spectra were
recorded as KBr pellets on a Nicolet 750 FTIR spectrophotometer.
Magnetic data were recorded using a Quantum Design SQUID
magnetometer. To avoid orientation in the magnetic field,the
samples were pressed in a home-made Teflon sample holder
equipped with a piston. The data were corrected for diamagnetism
of the constituent atoms using Pascal’s constants. The X-ray
diffraction measurements for 1 were collected on a Bruker Apex
SMART CCD system equipped with Mo Ka radiation
(λ= 0.71073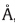 ) at room temperature. Cell constants and an
orientation matrix for data collection were obtained by leastsquares
refinement of the diffraction data from 7497 unique
reflections. The structure was solved by direct methods and refined
by a full matrix least squares technique based on F2 using the
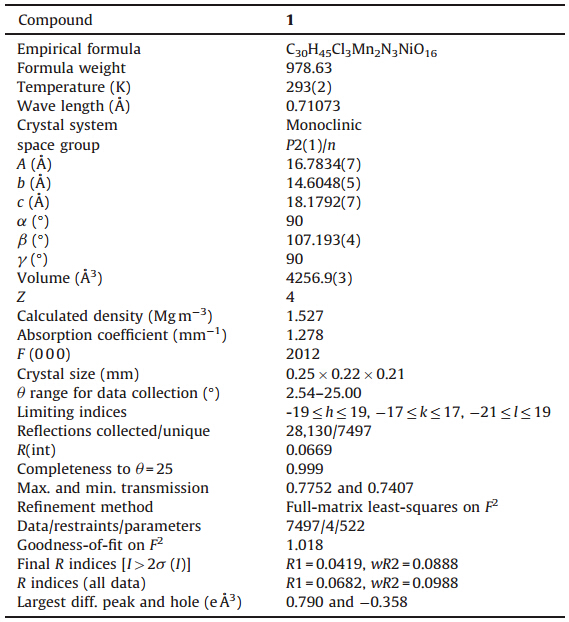
SHELX program [9]. The crystallographic data for the four
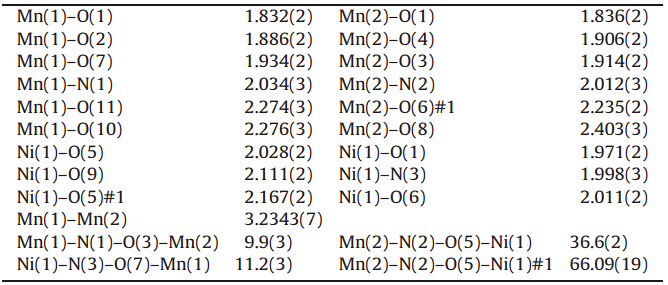
compounds are summarized in Table 1,and selected bond lengths
and torsion angles are list in Table 2.
) at room temperature. Cell constants and an
orientation matrix for data collection were obtained by leastsquares
refinement of the diffraction data from 7497 unique
reflections. The structure was solved by direct methods and refined
by a full matrix least squares technique based on F2 using the
SHELX program [9]. The crystallographic data for the four
compounds are summarized in Table 1,and selected bond lengths
and torsion angles are list in Table 2.
| Table 1 Crystal data and structure refinement of 1. |
| Table 2 Selected bond lengths ( 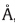 ) and torsion angles (°) for 1. ) and torsion angles (°) for 1. |
To pale pink solutions of Mn(ClO4)2·6H2O (1 mmol) in MeOH (20 mL) were added equivalent amounts of the Cl-H2Sao (1 mmol, 10 mL),and five times of Et3N (0.7 mL). After 30 min of stirring,the Ni(ClO4)2·6H2O (0.5 mmol) in MeOH (20 mL) was added. The solutions were left stirring for 30 min,filtered,and then left to slowly evaporate. Dark green single crystals suitable for X-ray diffraction study were obtained after 2 days. Elemental anal. calcd. (found) for 1 (formula C30H45Cl3Mn2N3NiO16): C 36.82 (36.75),H 4.64 (4.44),N 4.29 (4.34) %. ICP result calcd. (found): Mn,11.0 (11.2); Ni 6.2 (6.0) %. IR (KBr pellet) = 416.1 (m),466.6 (m),533.1 (w),626.8 (s),648.1 (s),679.5 (vs),749.5 (s),789.4 (w),827.8 (w), 856.8 (w),918.5 (vs),1026.1 (vs),1098.6 (m),1124.4 (m),1153.9 (m),1170.8 (m),1202.5 (s),1277.0 (vs),1327.8 (w),1392.5 (vs), 1440.0 (s),1471.4 (m),1527.9 (s),1597.5 (vs),3392.3 (s) cm-1.
3. Results and discussion
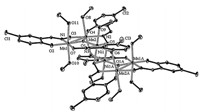
Single crystal X-ray diffraction analysis reveals that the
complex 1 (Fig. 1),like most Mn6-Sao systems,consists of two
off-set [Mn2NiO]6+ triangles lined together via two oximate oxygen
atoms from two η1:η2:η1:μ3 Cl-Sao2- ligands and two phenolate
oxygen atoms derived from two h2:h1:h1:m3 Cl-Sao2- ligands. The
remaining two oximato(-2) ligands each bridge one NiII ion in an
η1:η1:η1:μ fashion,thus forming a [MnIII
4Ni2(μ3-O)2(μ3-
ONR)2(μ-ONR)4(μ-OR0)2]4+ core. The remaining axial coordination
sites on the Mn and Ni ions are filled by methanol molecules. All
MnIII and NiII ions display a six-coordinate distorted octahedral
geometry,and the Jahn-Teller axes of all three ions are nearly
parallel to each other and are roughly perpendicular to the
[Mn2NiO]6+ plane. The oxidation states for the two manganese(III)
ions and nickel(II) ions were established by charge-valence
considerations,bond length distances,and bond valence sum
(BVS) [11] calculations with the obtained values of 3.24,3.22 and
2.16 for Mn1,Mn2 and Ni1,respectively. The nearest distance
between Mn ions different from Mn4Ni2 cores is 9.659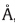 ,Mn-Ni
distance is 9.087
,Mn-Ni
distance is 9.087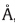 ,and Ni-Ni distance is above 10
,and Ni-Ni distance is above 10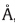 ,which
indicates that the inter-cluster magnetic interaction can be
neglected. Adjacent molecules are linked by intermolecular H
bonds to form a 1D infinite chain structure (Fig. 2),and there is no
obvious π-π stacking interaction between phenolate.
,which
indicates that the inter-cluster magnetic interaction can be
neglected. Adjacent molecules are linked by intermolecular H
bonds to form a 1D infinite chain structure (Fig. 2),and there is no
obvious π-π stacking interaction between phenolate.
|
Download:
|
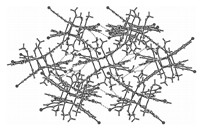
| Fig. 1.Perspective view of the complex 1 (30% thermal probability ellipsoids,for clarity hydrogen atoms and uncoordinated methanol molecules are omitted). | |
|
Download:
|
| Fig. 2.Molecular packing view of 1 along the b direction; the intermolecular hydrogen bonds and weak bonds are shown as dashed lines. | |
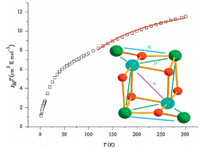
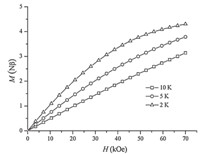
Solid-state DC (direct current) magnetic susceptibility (χM) data were collected in a 0.1 T field in the 1.8-300 K range (Fig. 3). The χMT value for 1 decreases steadily from 11.59 emu K mol-1 at 300 K to 5.47 emu K mol-1 at 35 K,and then decreases sharply to 1.14 at 1.8 K. The χMT value at 300 K for 1 is lower than 14 emu K mol-1,the value expected for a Mn4Ni2 complex with non-interacting metal centers between Mn and Ni ions. The decrease of the χMT value with decreasing temperature also indicates antiferromagnetic coupling within complex 1. The rapid decrease observed in the χMT value at 35-1.8 K range is most likely due to zero-field splitting effects and perhaps weak intermolecular interactions mediated by the hydrogen bonding in the crystal structure. The non-superposition of the M-H data (Fig. 4) on a single master curve suggests the presence of magnetic anisotropy and antiferromagnetic interaction on 1.
|
Download:
|
| Fig. 3.Plot of χMT vs. T for 1. The solid line represents the best fit of the experimental data. Insert: the interaction between Mn and Ni ions (red ball,oxygen; blue ball,nickel; green,manganese). | |
|
Download:
|
| Fig. 4.Plot of M Y. Zhang et al. / Chinese Chemical Letters 25 (2014) 937-940 939 vs. H for 1. | |
The magnetic susceptibilities are fitted by least-squares fitting procedure with Levenberg-Marquardt algorithm using the program MAGPACK [10].

AC magnetic susceptibility measurements were collected on complex 1 with an oscillating magnetic field of 3.5 Oe in the frequency range 10-1000 Hz and at temperatures between 1.8 and 6 K to probe for any slow magnetic relaxation associated withSMM behavior. No out-of-phase susceptibility ( χ") was observed for the complex 1 above 2 K. We speculated that the location of Ni ions may affect the SMM property of the cluster. If the Ni ions occupy the position of other two Mn ions,the cluster may experience magnetic relaxation at temperatures between 1.8 K and 6 K.
4. Conclusion
In summary,the bimetallic hexanuclear cluster based on 5- chlorosalicylaldehyde oxime has similar structure [M6O2] core to Mn6-Sao system. Magnetic investigation reveals that 1 exhibits an antiferromagnetism behavior. No SMMs properties were found in complex 1.
Supplementary materials
Crystallographic data for the structural analysis have been deposited with the Cambridge Crystallographic Data Center,CCDC 961675,for complex 1. These data can be obtained free of charge at www.ccdc.cam.ac.uk/conts/retrieving.html.
| [1] | (a) R. Bagai, G. Christou, The Drosophila of single-molecule magnetism:[Mn12O12(O2CR)16(H2O4], Chem. Soc. Rev. 38 (2009) 1011-1026;(b) K.Y. Bliokh, S.A. Gredeskul, P. Rajan, I.V. Shadrivov, Y.S. Kivshar, Nonreciprocal Anderson localization in magneto-optical random structures, Phys. Rev. B 85 (2012) 014205;(c) H.L. Cai, Y. Zhang, D.W. Fu, et al., Above-room-temperature magnetodielectric coupling in a possible molecule-based multiferroic:triethylmethylammonium tetrabromoferrate(III), J. Am. Chem. Soc. 134 (2012) 18487-18490;(d) J. Schwöbel,Y.S. Fu, J. Brede, et al., Real-space observationof spin-splitmolecular orbitals of adsorbed single-molecule magnets, Nat. Commun. 3 (2012) 953;(e) Y. Zhang, X.B. Han, Z.M. Zhang, Z.J. Liu, E.B. Wang, A {Ni7} cluster-containing sandwich-type phosphotungstate functionalized by organic bisphosphonate ligands and its two-dimensional supramolecular structure, Chin. Chem. Lett. 24 (2013) 581-584. |
| [2] | L. Bogani, W. Wernsdorfer, Molecular spintronics using single-molecule magnets, Nat. Mater. 7 (2008) 179-186. |
| [3] | R. Bircher, G. Chaboussant, C. Dobe, et al., Single-molecule magnets under pressure, Adv. Funct. Mater. 16 (2006) 209-220. |
| [4] | (a) T.N. Nguyen, W. Wernsdorfer, K.A. Abboud, G. Christou, A supramolecular aggregate of four exchange-biased single-molecule magnets, J. Am. Chem. Soc. 133 (2011) 20688-20691;(b) T. Taguchi, M.R. Daniels, K.A. Abboud, G. Christou, Mn4, Mn6, and Mn11 clusters from the use of bulky diphenyl(pyridine-2-yl)methanol, Inorg. Chem. 48 (2009) 9325-9335;(c) F. Haque, M. Langhirt, E. del Barco, T. Taguchi, G. Christou, Magnetic field dependent transport through a Mn4 single-molecule magnet, J. Appl. Phys. 109 (2011) 07B112. |
| [5] | (a) A.M. Ako, I.J. Hewitt, V. Mereacre, et al., A ferromagnetically coupled Mn19 aggregate with a record S=83/2 ground spin state, Angew. Chem. 118 (2006) 5048-5051;(b) G.E. Kostakis, A.M. Ako, A.K. Powell, Structural motifs and topological representation of Mn coordination clusters, Chem. Soc. Rev. 39 (2010) 2238-2271;(c) A.M. Ako, V. Mereacre, I.J. Hewitt, et al., Enhancing single molecule magnet parameters, synthesis, crystal structures and magnetic properties of mixed-valent Mn4 SMMs, J. Mater. Chem. 16 (2006) 2579-2586. |
| [6] | (a) R.T. Scott, S. Parsons, M. Murugesu, et al., Synthesis, structure and magnetic properties of a trinuclear [MnIIIMnII2] single-molecule magnet, Chem. Commun. (2005) 2083-2085;(b) J. Gómez-Segura, J. Veciana, D. Ruiz-Molina, Advances on the nanostructuration of magnetic molecules on surfaces: the case of single-molecule magnets (SMM), Chem. Commun. (2007) 3699-3707. |
| [7] | (a) C.J. Milios, R. Inglis, R. Bagai, et al., Enhancing SMM properties in a family of[Mn6] clusters, Chem. Commun. 33 (2007) 3476-3478;(b) L.F. Jones, A. Prescimone, M. Evangelisti, E.K. Brechin, 1D chains of Mn6 singlemolecule magnets, Chem. Commun. 15 (2009) 2023-2025;(c) E. Cremades, J. Cano, E. Ruiz, et al., Theoretical methods enlighten magnetic properties of a family of Mn6 single-molecule magnets, Inorg. Chem. 48 (2009) 8012-8019;(d) C.J. Milios, R. Inglis, A. Vinslava, et al., Toward a magnetostructural correlation for a family of Mn6 SMMs, J. Am. Chem. Soc. 129 (2007) 12505-12511;(e) A. Prescimone, C.J. Milios, S. Moggach, et al., [Mn6] under pressure: a combined crystallographic and magnetic study, Angew. Chem. Int. Ed. 47 (2008) 2828-2831. |
| [8] | (a) E. Cremades, C.D. Pemmaraju, S. Sanvito, E. Ruiz, Spin-polarized transport through single-molecule magnet Mn6 complexes, Nanoscale 5 (2013) 4751-4757;(b) M. Haryono, M. Kalisz, R. Sibille, et al., One dimensional assembly of Mn6 single molecule magnets linked by oligothiophene bridges, Dalton Trans. 39 (2010) 4751-4756. |
| [9] | (a) G.M. Sheldrick, SHELX 97, Program for Crystal Structures Refinement, University of Göttingen, Germany, 1997;(b) G.M. Sheldrick, SHELX 97, Program for Crystal Structures Solution, University of Göttingen, Germany, 1997. |
| [10] | J.J. Borrás-Almenar, J.M. Clemente-Juan, E. Coronado, B.S. Tsukerblat, Highnuclearity magnetic clusters: generalized spin hamiltonian and its use for the calculation of the energy levels, bulk magnetic properties, and inelastic neutron scattering spectra, Inorg. Chem. 38 (1999) 6081-6088. |
| [11] | N.E. Brese, M. O'Keeffe, Bond-valence parameters for solids, Acta Crystallogr. B 47 (1991) 192-197. |