b Guangzhou Key Laboratory of Materials for Energy Conversion and Storage, South China Normal University, Guangzhou 510006, China
Stannous oxides,such as rutile SnO2 and tetragonal SnO,have received great attention as functional materials,such as catalysts [1, 2],gas sensors [3, 4],solar cells [5],optoelectronic devices [6, 7], and electrode materials of lithium ion batteries [8, 9, 10],and SnO can be oxidized to SnO2 at elevated temperature easily [11].Synthesis of nano-materials with controlled morphology,size and crystal structure is a key step toward nano-technological applications [12]. Particularly,the novel three-dimensional (3D) hierarchical architectures that are assembled by 1D or 2D nanoscale units,have attracted considerable attention because of their improved performance,such as larger surface area,more efficient catalytic activity,superior electrochemical performance and structural stability [12, 13, 14, 15]. To date,a variety of SnO2 2D or 3D structures have been achieved,such as SnO2 nanorods [16], single-layered hollow microspheres and flower like nanospheres have been synthesized through a facile template-free hydrothermal method with the help of PEG400 [17]. Hierarchical SnO2 flower-like architectures consisting of numerous aggregative nanosheets have been successfully synthesized via a facile hydrothermal method with the help of PEG and subsequent calcinations [18]. Square SnO2 nano-sheets have been synthesized by a template-free hydrothermal method based on the reaction between SnCl2 and NaOH in ethanol/water [19] and so on. Certainly,many 1D and 2D SnO distinct morphologies have been successfully synthesized such as ultrathin SnO nanosheets [20], single crystalline SnO nanoplatelets [9],SnO disk-like structures [21],tetragonal SnO nanobranch [22]. Recently some 3D SnO structures have been synthesized in a water system. For example, well-controlled novel architectures of SnO with 3D flowerlike microstructures were synthesized in an aqueous solution using SnCl2.2H2O and NaOH [23],and novel self-assembled 3D hierarchical polygon shaped structures of SnO were synthesized with a template-free hydrothermal method using SnCl2.2H2Oand KOH in water [10, 24]. Compared to SnO2 ,there have fewer reports in the preparation of novel SnO 3D structures,especially in the ethanol/water system.
In this letter,a hierarchical flower-like SnO consisting of various microsheets was successfully synthesizedviaa template-free and surfactant-free hydrothermal process based on the reaction between SnCl2 and NaOH in different ratios of ethanol and water. A possible growth mechanism of the 3D structure is discussed. Furthermore,the effect of ethanol and NaOH on the formation of SnO and SnO2 is discussed too. 2. Experimental
SnCl2.2H2O and NaOH were of analytical grade,and needed no
further purification. Firstly,35 mmol of NaOH was dissolved in
different volume ratios (mL/mL) of ethanol/water with 27:13 2:1
(symbolized as Sample A),20:20 = 1:1 (Sample B),10:30 = 1:3
(Sample C) at room temperature,respectively. Then 12 mmol of
SnCl2.2H2O was dropped into each solution under continuous
magnetic stirring for another 0.5 h. The obtained mixtures were
transferred into a 150 mL Teflon-lined stainless autoclave,sealed
and maintained at 180°C for 12 h,and then cooled to room
temperature. The obtained precipitates were centrifuged and
washed several times with water and ethanol,respectively,until Cl-ions could not be detected. The products were finally dried in
the vacuum at 70°C for 16 h. As comparison,a same synthesis was
performed as Sample B but no addition of NaOH was performed,
which was symbolized as Sample D.
2:1
(symbolized as Sample A),20:20 = 1:1 (Sample B),10:30 = 1:3
(Sample C) at room temperature,respectively. Then 12 mmol of
SnCl2.2H2O was dropped into each solution under continuous
magnetic stirring for another 0.5 h. The obtained mixtures were
transferred into a 150 mL Teflon-lined stainless autoclave,sealed
and maintained at 180°C for 12 h,and then cooled to room
temperature. The obtained precipitates were centrifuged and
washed several times with water and ethanol,respectively,until Cl-ions could not be detected. The products were finally dried in
the vacuum at 70°C for 16 h. As comparison,a same synthesis was
performed as Sample B but no addition of NaOH was performed,
which was symbolized as Sample D.
Structural characterizations of the samples were performed by
X-ray diffraction (XRD,Model Y2000,China) with Cu Karadiation
( = 1.5406
= 1.5406 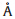 ) at 30 kV and 20 mA. The structure and morphology
of the products were studied using a scanning electron microscope
(SEM,Model ZEISS Ultra 55,Germany).
3. Results and discussion
) at 30 kV and 20 mA. The structure and morphology
of the products were studied using a scanning electron microscope
(SEM,Model ZEISS Ultra 55,Germany).
3. Results and discussion
Fig. 1 shows typical XRD patterns of the SnOx synthesized in different volume ratios of ethanol/water with or without NaOH. Almost all the diffraction peaks in Fig. 1(A-C) are well indexed to the tetragonal SnO standard patterns JCPDS 06-0395 except that a small peak at around 26.958in Fig. 1(B) and (C),which is identified to the intermediate Sn6O4(OH)4 [23, 25]. Compared with the standard patterns,the intensity of (0 0 2 ) crystal plane is obviously strengthened and (1 0 1) and (1 1 0) planes are significantly depressed for the three samples. There were no descriptions of similar results in water systems at different temperature and time [10, 23, 24, 25],indicating that ethanol favors the crystal growing toward (0 0 1) crystal planes judged by the intense peaks of the (0 0 1) and (0 0 2) diffractions in the XRD patterns (Fig. 1A-C). The facts that the XRD diffraction intensity of SnO was reduced with the decrease of ethanol amount and the intermediate Sn6O4(OH)4 appeared in sample B and C indicate that ethanol might have enhanced the crystal size and the degree of crystallinity. The XRD patterns of sample D (Fig. 1D) reveal that all the diffraction peaks index to the rutile SnO2 (JCPDS 41-1445),and the broadened peaks indicate the crystal size of SnO2 is reasonably small. Compared with the results obtained from sample B,NaOH should have played a crucial role in the formation of SnOx.

|
Download:
|
| Fig. 1. XRD patterns of samples prepared at different volume ratios of ethanol/water with NaOH: (A) 2:1; (B) 1:1; (C) 1:3; and (D) 1:1 without NaOH | |
Figs. 2 and 3 display the SEM images of the samples at low and high magnification. One can see the morphology of the samples is markedly different from each other. When the volume ratio of ethanol/water was 2:1,several rose flower-like hierarchical 3D architectures with a diameter of about 40mm formed uniformly. Each flower was assembled regularly by a large number of microsheets (Fig. 2a). The enlarged image of an individual architecture in Fig. 3a shows that the unique flower-like hierarchical architecture is regularly composed of numerous well-ordered thin microsheets with an octodecahedral shape. The octodecahedral sheet is composed of two irregular octagons at the bottom and the top,respectively,and sixteen trapezoids two of which have a communal edge at the sides (the front view of the sheets was inserted in Fig. 3a according to the morphology in red circle and rectangle in Fig. 3a). The irregular octagons at the bottom and the top are about 15mm for four long edges and 2-3mm for the other four short edges,and the thickness of the octodecahedral sheet is about 3mm. These microsheets grow in an unexpected fashion in that the flowers bloom naturally and form an open porous structure. Additional high-magnification images reveal the surface of the sheets is rough and composed of numerous smaller nanoparticles (Fig. 3b). As the ratio of ethanol/water was decreased to 1:1,the flower-like structure disappeared and the SnO changed to irregular blocks as a base with about 20-50mm of each edge and 5-10mm of thickness,and many thin nanosheets anchored on the base (Fig. 2b). These thin microsheets are irregular and 10-100 nm in thickness with poor dispersion (Fig. 3c). Additional magnified images illustrate that numerous nanoparticles formed the rough surface of the sheets (Fig. 3d),which is similar to that of Sample A. It is interesting that as the ratio of ethanol/water was decreased to 1:3,most of SnO self-assembled into more regularquasisquare blocks with 20-40mm in length and 2-5mm in thickness as a base, and many nanosheets anchored on the surface of thequasisquare blocks uniformly (Fig. 2c). These nanosheets have good dispersion and form many porous among them (Fig. 3e). In a high magnification image,the sheets seem very thin and smooth,and with about 20 nm in thickness and 1000 nm in length (Fig. 3f). For sample D synthesized without NaOH in Fig. 2d,SnO2 nano-spheres of 200 nm in diameter are obtained,and the spheres are composed of numerous SnO2 nanoparticles with smaller than 10 nm diameters (Fig. 3g). Here NaOH is the key factor to the formation of SnOx. SnO2 can form directly by the hydrolysis reaction (1) at room temperature and hydrothermal oxidation (2) without NaOH in solution [25]:
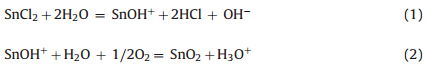
While with sufficient amount of NaOH,the hydrolysis reactions are as follows:
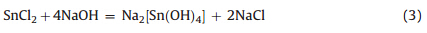
And then,in the hydrothermal process,Na2[Sn(OH)4] transformed into SnO crystals: The total reaction is: Uchiyama reported that the threshold pH values for the
formation of SnO2 and SnO were 3.3 and 13.1,respectively [25].
In Ref. [23],multi-architectured SnO formed by platelets at
30°C through a reaction between SnCl2.2H2O and NaOH in water.
There are only limited numbers of large microsheets (25mmin
length and 1mm in thickness) crossing each other,and all sheet
edges are rough and possess multilayers. As the temperature
increased,the thickness of the microsheets decreased to thin
nanosheets and many closely attached nanosheets with different
orientations assembling the 3D structures at 70-90°C. The results
showed that the cross sheet structures can form at room
temperature (no hydrothermal process) in a water system. In
Refs. [24, 26],SnCl2.2H2O was hydrolyzed in water with KOH or
NaOH and followed by a hydrothermal process,a SnO 3D structure
where many thin microsheets piled up to 8-12mm was achieved.
Some nanosheets anchored on the big base block if the reaction
was prolonged,which is very similar to the structure of sample B
and C in this study. Compared the references [23, 24, 26] with this
study,one can deduce that the high temperature and hydrothermal process is a delamination process of the SnO base block. The
thick and rough SnO blocks formed at room temperature can be
delaminated by a high temperature hydrothermal process to
numerous microsheets or nanosheets. Here,a possible mechanism
for the formation of the flower like SnO 3D structure an in ethanol/
water system is proposed based on the structure and morphology
of the samples A,B,C and those reported in the literature. The
schematic illustration is shown in Fig. 4.
In a typical growth mechanism the formation of square blocks
occurs at the beginning of the reaction in an ethanol/water = 1:3
system (Sample C,the ethanol amount is small and SnCl2
hydrothermal environment is similar to that of the pure water
system [24]),after a long (12 h) hydrothermal process at high
temperature (180°C),these blocks were delaminated into thin and
smooth quasisquare nanosheets and anchored on the residual
blocks. As the ratio of ethanol/water increased to 1:1 (Sample B),
the polarity and dielectric constant of the solvent would be
decreased,which resulted in a slower hydrolysis of SnCl2 and more
irregular growth of the microsheets. The slow hydrolysis will result
in larger crystal nucleus and the formation of rougher surface of
the microsheets (Fig. 3d) than that of sample C. In addition,ethanol
will influence the morphology and the delamination of the base
blocks. Compared with Sample C where most of the base blocks
and the peeled nanosheets are of regularquasisquare shape,the
irregular blocks were peeled and cracked into irregular microsheets,which anchored on the blocks disorderly and nonuniformly. As the ethanol/water ratio increased to 2:1,the SnCl2
hydrolysis rate was reduced further,and the larger SnO crystals
formed the roughest surface of the sheets (Fig. 3b). The large ratio
of ethanol accelerates the delamination of the blocks and lead to
few large base blocks. Instead,a large number of uniform
octodecahedral microsheets formed and the sheets self-assembled
as the rose flower-like structures by a hydrothermal process. As a
result,abundant surface state and some defects are created in
these highly dispersive 2D microsheets and 3D hierarchical
structures. In Ref. [19],ethanol/water systems with a small
amount of NaOH (pH<13) were used to synthesize SnO2 by a
hydrothermal method. The results showed that the size and
morphology of the nanosheets also changed with the ratio of
ethanol/water. The ethanol content is a crucial factor for the
formation of the SnO flower like structures. Further investigation
of the formation mechanism of SnOx
will be the next step.
4. Conclusion
Novel rose flower-like SnO 3D hierarchical structures consisting
of well-ordered microsheets were synthesized by a simple
hydrothermal method in an ethanol/water system. The size and
morphology of microsheets can be controlled by changing the
ethanol/water ratio. The ratio of ethanol in the solvent plays an
important role in the formation of hierarchical flower-like SnO
architectures and the NaOH content is critical for the formation of
SnO and SnO2 in this system.
Acknowledgment
This study was financially supported by Guangdong Natural
Science Foundation (No. S2011010005788).
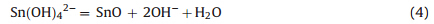


Fig. 2. SEM images of (a) sample A (ethanol/water = 2:1 with NaOH), (b) sample B (ethanol/water = 1:1 with NaOH), (c) sample C (ethanol/water = 1:3 with NaOH) and(d)
sample D (ethanol/water = 1:1 without NaOH) at low-magnification.

Fig. 3. SEM images of the sample A (a and b), sample B (c and d), sample C (e and f) and sample D (g) at different magnification.

Fig. 4. Growth mechanism flow chart of flower-like SnO 3D structures in ethanol/water system.
| [1] | K. Shimizu, M. Katagiri, S. Satokawa, A. Satsuma, Sintering-resistant and selfregenerative properties of Ag/SnO2 catalyst for soot oxidation, Appl. Catal. B 108/109 (2011) 39-46. |
| [2] | J. Zhao, L. Ma, X.L. Xu, F. Feng, X.N. Li, Synthesis of carbon-supported Pd/SnO2 catalyst for highly selective hydrogenation of 2,4-difluoronitrobenzene, Chin. Chem. Lett. (2014), http://dx.doi.org/10.1016/j.cclet.2014.01.024. |
| [3] | W. Zeng, B. Miao, Q. Zhou, L.Y. Lin, Hydrothermal synthesis and gas sensing properties of variety low dimensional nanostructures of SnO2, Physica E 47 (2013) 116-121. |
| [4] | V.X. Hien, J.H. Lee, J.J. Kim, Y.W. Heo, Structure, NH3 sensing properties of SnO thin film deposited by RF magnetron sputtering, Sens. Actuators B 194 (2014) 134-141. |
| [5] | J.Y. Liu, T. Luo, S.T. Mouli, et al., A novel coral-like porous SnO2 hollow architecture: biomimetic swallowing growth mechanism and enhanced photovoltaic property for dye-sensitized solar cell application, Chem. Commun. 46 (2010) 472-474. |
| [6] | Y.F. Wang, B.X. Lei, Y.F. Hou, et al., Facile fabrication of hierarchical SnO2 microspheres film on transparent FTO glass, Inorg. Chem. 49 (2010) 1679-1686. |
| [7] | V.G. Kravets, Spectroellipsometric and photoluminescent studies of SnOx nanostructures doped with Sm ions, J. Alloys Compd. 509 (2011) 8888-8893. |
| [8] | X.M. Yin, L.B. Chen, C.C. Li, et al., Synthesis of mesoporous SnO2 spheres via selfassembly and superior lithium storage properties, Electrochim. Acta 56 (2011) 2358-2363. |
| [9] | Y.J. Hu, K.X. Xu, L.Y. Kong, et al., Flame synthesis of single crystalline SnO nanoplatelets for lithiuμ-ion batteries, Chem. Eng. J. 242 (2014) 220-225. |
| [10] | M.Z. Iqbal, F.P. Wang, H.L. Zhao, et al., Structural and electrochemical properties of SnO nanoflowers as an anode material for lithium ion batteries, Scripta Mater. 67 (2012) 665-668. |
| [11] | K.M. Li, Y.J. Li, M.Y. Lu, C.L. Kuo, L.J. Chen, Direct conversion of single-layer SnO nanoplates to multi-layer SnO2 nanoplates with enhanced ethanol sensing properties, Adv. Funct. Mater. 19 (2009) 2453-2456. |
| [12] | X. Wang, Y.J. Yang, Y. Ma, J.N. Yao, Controlled synthesis of multi-shelled transition metal oxide hollow structures through one-pot solution route, Chin. Chem. Lett. 24 (2013) 1-6. |
| [13] | L.P. Qin, J.Q. Xu, X.W. Dong, et al., The template-free synthesis of square-shaped SnO2 nanowires: the temperature effect and acetone gas sensors, Nanotechnology 19 (2008) 185705-185712. |
| [14] | C. Goebbert, M.A. Aegerter, D. Burgard, R. Nass, H. Schmidt, Ultrafiltration conducting membranes and coatings from redispersable, nanoscaled, crystalline SnO2:Sb particles, J. Mater. Chem. 9 (1999) 253-258. |
| [15] | H.J. Zhang, Q.Q. He, X.D. Zhu, et al., Surfactant-free solution phase synthesis of monodispersed SnO2 hierarchical nanostructures and gas sensing properties, CrystEngComm 14 (2012) 3169-3176. |
| [16] | X.W. Huang, Z.J. Liu, Y.F. Zheng, Q.L. Nie, Synthesis of SnO2 nanorods from aqueous solution: the effect of preparation conditions on the formed patterns, Chin. Chem. Lett. 21 (2010) 999-1002. |
| [17] | X.H. Zhang, M.X. Huang, Y.J. Qiao, Synthesis of SnO2 single-layered hollow microspheres and flower like nanospheres through a facile template-free hydrothermal method, Mater. Lett. 95 (2013) 67-69. |
| [18] | Q.Y. He, W. Zeng, Y. Wang, et al., Large scale synthesis of flower-like SnO2 nanostructures via a facile hydrothermal route, Mater. Lett. 113 (2013) 42-45. |
| [19] | Y. Li, Y.Q. Guo, R.Q. Tan, et al., Synthesis of SnO2 nano-sheets by a template-free hydrothermal method, Mater. Lett. 63 (2009) 2085-2088. |
| [20] | G. Sun, N.T. Wu, Y.W. Li, et al., Solvothermal synthesis and characterization of ultrathin SnO nanosheets, Mater. Lett. 98 (2013) 234-237. |
| [21] | P.H. Sumana, A.A. Felix, H.L. Tuller, J.A. Varelaa, M.O. Orlandia, Giant chemoresistance of SnO disk-like structures, Sens. Actuators B 186 (2013) 103-108. |
| [22] | J.H. Shin, J.Y. Song, Y.H. Kim, H.M. Park, Low temperature and self-catalytic growth of tetragonal SnO nanobranch, Mater. Lett. 64 (2010) 1120-1122. |
| [23] | Y. Liang, H.W. Zheng, B. Fang, Synthesis and characterization of SnO with controlled flower like microstructures, Mater. Lett. 108 (2013) 235-238. |
| [24] | M.Z. Iqbal, F.P. Wang, T. Feng, et al., Facile synthesis of self-assembled SnO nanosquare sheets and hydrogen absorption characteristics, Mater. Res. Bull. 47 (2012) 3902-3907. |
| [25] | H. Uchiyama, H. Ohgi, H. Imai, Selective preparation of SnO2 and SnO crystals with controlled morphologies in an aqueous solution system, Cryst. Growth Des. 6 (2006) 2187-2190. |
| [26] | F.I. Pires, E. Joanni, R. Savu, et al., Microwave-assisted hydrothermal synthesis of nanocrystalline SnO powders, Mater. Lett. 62 (2008) 239-242. |




