Fuel cells have attracted great attention recently due to the depletion of fossil fuels as well as the worsening environmental situation. Among different types of fuel cells,proton exchange membrane fuel-cells are of great interest because of their low pollutant emission,high energy density,and ease of liquid fuel handling,and so they are promising candidates for portable power sources [1, 2, 3, 4]. However,there are still some critical obstacles inhibiting broad applications of proton exchange membrane fuelcells,including low electrocatalytic activity of anode catalysts for methanol oxidation reaction and the high cost of noble metal platinum (Pt)-based catalysts [5, 6, 7].
Pt-based nanocrystals (NCs) are being explored as an alternative to single Pt with the addition of a second or third metal (e.g.Pd, Cu,Au,Feetc.) to Platinum to form Pt-based alloy catalysts for electrocatalysis [8, 9, 10, 11, 12]. The success in electrocatalysis of these alloy materials has been attributed to the bi-functional mechanism and the electronic effect induced by the electronic interaction of Pt with other metals. Therefore,it is necessary to investigate the catalytic activity of the Pt-based alloy catalysts toward favorable kinetics and better selectivity of Co2.
The first-row transition metals (FRTM) have been widely investigated at the nanoscale as catalytic materials because of their potential activities and relatively low costs. However,nano-FRTM are easily etched by acidic solution. When FRTM were alloyed with noble metals,their stabilities under acidic condition could be enhanced [13, 14, 15]. Moreover,the incorporation of FRTM into the noble metals alloy structure may not only lead to the enhancement of the catalytic performance,but also reduce the consumption of the noble metals [15, 16, 17, 18]. Different processes,including electroless plating,a water-in-oil microemulsion,and polyol method,have been employed to prepare bimetallic PtCo catalysts which exhibited improved catalytic activity in the anode oxidation reaction [19, 20, 21, 22].
Improving the catalytic activity of anode catalysts is an important task in direct methanol and formic acid fuel cell development. In the present work,we report the synthesis, characterization,and electro-oxidation properties of Pt0.35Pd0.35Co0.30/C catalysts,which exhibited excellent electrocatalytic activity in methanol and formic acid electro-oxidation. 2. Experimental
2.1. Preparation of catalyst
The carbon supported nanocatalysts (20 wt%) of the different ratios (Pt:Pd:Co) of the ternary system,Pt0.65Co0.35/C,Pd0.65Co0.35/C, Pt/C,and Pd/C were prepared by the NaBH4-reduction scheme. Typically,for preparation Pt0.35Pd0.35Co0.30/C,10 mL of K2PtCl6 (446 mg),Na2PdCl4(270 mg) and CoCl2.6H2O (93 mg) were mixed with 40 mL of aqueous solution containing well-dispersed Vulcan XC-72 carbon (120 mg,500 m2g-1). Then,the fresh NaBH4 aqueous solution (10 mL,0.6 mol L-1) was added to the above mixture under magnetic stirring in nitrogen atmosphere. After 2 h, Black metal catalyst particles were then isolated by centrifugation and washed with plenty of double distilled water. The synthesized catalysts were dried in an oven at 80°C for 12 h. The product Pt0.35Pd0.35Co0.30/C was obtained and ready for catalytic use in methanol and formic acid oxidation reactions. 2.2. Instrument and electrochemical measurement
X-ray diffraction (XRD) analysis was carried out on Bruker D8-ADVANCE diffractometer with Cu Karadiation of wavelength
 = 0.15418 nm. Transmission electron microscopy (TEM,JEOL
JEM-2100) was applied to characterize the morphology. The
analysis of the composition of the catalyst was obtained with an
inductively coupled plasma-atomic emission spectrometer (ICPAES; USA Thermo Jarrell-Ash Corp. ICP-9000(N+M)).
= 0.15418 nm. Transmission electron microscopy (TEM,JEOL
JEM-2100) was applied to characterize the morphology. The
analysis of the composition of the catalyst was obtained with an
inductively coupled plasma-atomic emission spectrometer (ICPAES; USA Thermo Jarrell-Ash Corp. ICP-9000(N+M)).
The electrochemical experiments were carried out with a CHI 660D electrochemical workstation (CH Instruments). A conventional three-electrode system was used with a modified glassy carbon (GC) electrode (3 mm in diameter) as the working electrode,a coiled platinum wire as the auxiliary electrode,and a leak-free Ag/AgCl (in saturated KCl) electrode was used as the reference electrode. A catalyst ink was prepared by taking an appropriate amount of catalyst in 5 wt% Nafion ionomer (Eletrochem. Inc.,USA) and 2-propanol,and the mixture was sonicated for 30 min. 3. Results and discussion
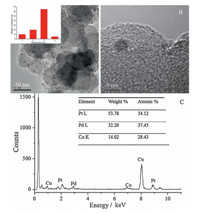
Pt:Pd:Co ternary alloy nanoparticles with different molar ratios were prepared and supported on carbon black,among which the Pt0.35Pd0.35Co0.30/C exhibited the highest catalytic activity in methanol and formic acid electro-oxidation. The XRD pattern of Pt0.35Pd0.35Co0.30/C was shown in Fig. 1,indicating the facecentered cubic (fcc) structure. Because the atomic radii of Co,Pt and Pd are 0.125 nm,0.139 nm and 0.137 nm,respectively [23], and according to the Hume-Rothery rule [24],the relative differences (δ=(rA -rB)/rA) of the atomic radii of Co to Pt and Pd are 10.1% and 8.8%,respectively,both lower than 15.0%,Co atoms can thus be incorporated into the Pt and Pd lattice to form a stable alloy structure. Moreover,neither a Pt nor a Pd single component peak was detected,confirming the presence of only one alloy structure,which was proved by transmission electron microscopy (TEM). In the typical TEM image of the Pt0.35Pd0.35Co0.30/C (Fig. 2A),it could be seen that Pt0.35Pd0.35Co0.30 NCs were well-dispersed on the XC-72 support,with an average particle size of about 3 nm (Inset in the Fig. 2A). Energy dispersive X-ray spectrometry (EDS) on one particle (Fig. 2B) displayed presence of Pt,Pd and Co (Fig. 2C) with the mole ratio of Pt:Pd:Co = 34.12:37.45:27.43,which agreed well with the value (Pt:Pd:Co = 0.35:0.37:0.28) obtained by inductively coupled plasma-atomic emission spectrometry (ICP-AES),which coincides with the theoretical value (Pt:Pd:Co = 35:35:30). It may be due to enhanced stability of Co under acidic condition. This can be proved by the next EDS of Pt0.35Pd0.35Co0.30/C after 100 cycles of cyclic voltammetry (CV) in 0.5 M H2SO4 solution.

|
Download:
|
| Fig. 1. XRD pattern of Pt0.35Pd0.35Co0.30/C, standard Pt, Pd and Co. | |
|
Download:
|
| Fig. 2. (A) and (B) TEM and HR-TEM of Pt0.35Pd0.35Co0.30/C. (C) EDS and the corresponding metal ratios. Inset of (A) the average particle size. | |
Moreover,Fig. 3A-C show the X-ray photoelectron spectroscopy (XPS) spectra around the Pt 4f,Pd 3d and Co 2p regions in Pt0.35Pd0.35Co0.30/C. For comparison,the Pt 4f,Pd 3d and Co 2p regions in Pt/C,Pd/C and Co/C are shown in Fig. 3D-F. The XPS results (Fig. 3A,B,D and E) shows that the binding energies for Pt 4f and Pd 3d in Pt0.35Pd0.35Co0.30/C are both shifted to lower values compared with those in Pt/C and Pd/C,respectively. Whereas the binding energy for Co 2p in Pt0.35Pd0.35Co0.30/C is shifted to a higher value relative to those in Co/C (Fig. 3C and F). These shifts demonstrate that some electrons are transferred from Co to Pt and Pd atoms in the alloy structure of Pt0.35Pd0.35Co0.30/C [25]. It can be easily understood that,in the present alloy structure,electrons can be transferred from atoms of Co to Pt and Pd to equilibrate the Fermi level because of the difference of the work functions of Pt(1 1 1) (6.13 eV),Pd(1 1 1) (5.67 eV) and Co(1 1 1) (5.44 eV) [26].

|
Download:
|
| Fig. 3. (A) Pt 4f, (B) Pd 3d, (C) Co 2p XPS spectra for Pt0.35Pd0.35Co0.30/C, (D) Pt 4f XPS spectra for Pt/C, (E) Pd 3d XPS spectra for Pd/C, and (F) Co 2p XPS spectra for Co/C | |
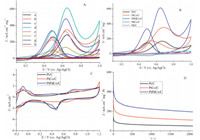
The catalytic activity of CVs for methanol electro-oxidation of Pt:Pd:Co ternary alloy nanoparticles with different molar ratios is shown in Fig. 4A,among which the Pt0.35Pd0.35Co0.30/C exhibited the highest catalytic activity. For comparison,the catalytic activity of Pt0.65Co0.35/C,Pt/C,Pd0.65Co0.35/C,and Pd/C were tested. Fig. 4B shows CVs scan of methanol oxidation of Pt0.35Pd0.35Co0.30/C, Pt0.65Co0.35/C,Pd0.65Co0.35/C,Pt/C and Pd/C in 0.5 mol L-1H2SO4 with 0.5 mol L-1CH3OH solution in the potential range of 0.1 V to 1.0 V vs.Ag/AgCl. The current peak normalized to the loading amount of Pt in the electrode. It is obvious that the Pd0.65Co0.35/C and Pd/C show no activity at all. The onset potential for the Pt0.35Pd0.35Co0.30/C catalyst was more negative than those of Pt0.65Co0.35/C and Pt/C catalysts,indicating good electrocatalytic activity. The If/Ib values for Pt0.35Pd0.35Co0.30/C (1.85vs.1.15 on Pt/C) implicated that methanol molecules can be more effectively oxidized on Pt0.35Pd0.35Co0.30/C during the forward potential scan, generating relatively less poisoning species as compared to Pt/C.
|
Download:
|
| Fig. 4. (A) Cyclic voltammetrys (CVs) of (a) Pt0.50Pd0.50/C, (b) Co0.1Pt0.45Pd0.45/C, (c) Co0.2Pt0.4Pd0.4/C, (d) Co0.3Pt0.35Pd0.35/C, (e) Co0.4Pt0.3Pd0.3/C, (f) Co0.5Pt0.25Pd0.25/C, (g) Co0.7Pt0.15Pd0.15/C, and (h) Co0.9Pt0.05Pd0.05/C in methanol electro-oxidation. (B) CVs profiles of Pt0.35Pd0.35Co0.30/C, Pt0.65Co0.35/C, Pd0.65Co0.35/C, Pd/C and Pt/C catalysts in 0.5 mol L -1 H2SO4+ 0.5 mol L -1 CH3OH solutions. (C) CVs profiles of Pt0.35Pd0.35Co0.30/C, Pt0.65Co0.35/C and Pt/C in a 0.5 mol L -1 H2SO4solution. (D) Chronoamperometry (CAs) of Pt0.35Pd0.35Co0.30/C, Pt0.65Co0.35/C and Pt/C was recorded at 0.6 V for 2100 s. | |
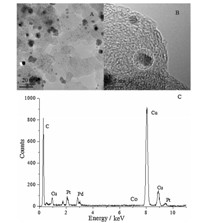
From Fig. 4B,we know that the Pt atoms are the crucial active sites in all the prepared catalysts in methanol electro-oxidation. Without Pt addition,Pd0.65Co0.35/C and Pd/C show no activity at all.Fig. 4C shows the CVs of the Pt0.35Pd0.35Co0.30/C,Pt0.65Co0.35/C and Pt/C catalysts measured in a nitrogen-saturated 0.5 mol L-1H2SO4 solution. The three catalysts exhibited hydrogen adsorption/ desorption peaks in the potential range from -0.2 V to 0.1 V. The electrochemical active surface area (ECSAs) estimated from the hydrogen adsorption peaks were 118.1 m2g-1for Pt0.35Pd0.35Co0.30/C NPs,90.3 m2g-1for Pt0.65Co0.35/C,respectively, which were higher than that of the Pt/C catalyst (82.1 m2g-1). Fig. 5 shows the TEM and EDS of Pt0.35Pd0.35Co0.30/C after 100 cycles. The molar ratio of Pt:Pd:Co of Pt0.35Pd0.35Co0.30/C measured by EDS was 0.40:0.33:0.27 after 100 cycles,indicating the relative high stability of Pt0.35Pd0.35Co0.30/C catalyst. To further explore the observed enhancement of the electrocatalytic activity and COtolerance,we carried out a chronoamperometry (CA) experiment under short time for Pt0.35Pd0.35Co0.30/C,Pt0.65Co0.35/C and Pt/C, shown in Fig. 4D for 2100 s at a constant potential of 0.6 Vvs.Ag/ AgCl. From Fig. 4D,CA curves indicated that the current density of Pt0.35Pd0.35Co0.30/C catalyst was higher than that of Pt0.65Co0.35/C and Pt/C catalyst. The much higher tolerance of the Pt0.35Pd0.35Co0.30/C catalyst can be attributed to the change of electronic structure induced by the Pt0.35Pd0.35Co0.30alloy.
|
Download:
|
| Fig. 5. (A)–(C) TEM, HR-TEM and EDS of Pt0.35Pd0.35Co0.30/C after 100 cycles of CVs in 0.5 mol L-1H2SO4solution. | |
The electro-catalytic activities of the catalysts were also evaluated in 0.5 mol L-1HCOOH + 0.5 mol L-1H2SO4 . Fig. 6A shows the results of electro-oxidation activities of Pt:Pd:Co with different ratios,and the Pt0.35Pd0.35Co0.30/C catalyst exhibited higher activity in formic acid oxidation. Recent studies revealed that Pd can catalyze the formic acid oxidation at the anode of polymer electrolyte membrane fuel cell [27]. Fig. 6B shows CVs scan of formic acid oxidation of Pt0.35Pd0.35Co0.30/C,Pt0.65Co0.35/C, Pd0.65Co0.35/C,Pt/C and Pd/C in 0.5 mol L-1H2SO4 with 0.5 mol L-1HCOOH solution. It is clear from Fig. 6B that the oxidation current of Pt0.35Pd0.35Co0.30/C,Pd0.65Co0.35/C and Pd/C for formic acid in the forward is higher than that for reverse scan which reflects the higher activity. During the forward scan,the catalyst of Pt0.65Co0.35/C showed a very small peak at about -0.14 V due to the hydrogen oxidation. At higher potentials,the current further increases at 0.50 V and reaches another peak at 0.68 V. The peak could be attributed to the oxidation of adsorbed CO and formic acid on available active sites recovered by CO removal [28]. In the negative potential scan,the peak appears at 0.35 V,which are much higher than those in the positive scan. The same behavior was found for formic acid oxidation on Pt/C. So,Pt0.65Co0.35/C and Pt/C show weak catalytic activities. From Fig. 6B,it is obvious that the Pd atoms are the crucial active sites in formic acid electrooxidation. To obtain steady state catalytic activity,CA measurements were conducted at 0.35 V for Pt0.35Pd0.35Co0.30/C,Pd0.65Co0.35/C and Pd/C (Fig. 6C). The results showed that the Pt0.35Pd0.35Co0.30/C shows the highest activity in formic acid electro-oxidation.

|
Download:
|
| Fig. 6. (A) Current density of formic acid electro-oxidation of (a) Pt0.50Pd0.50/C, (b) Co0.1Pt0.45Pd0.45/C, (c) Co0.2Pt0.4Pd0.4/C, (d) Co0.3Pt0.35Pd0.35/C, (e) Co0.4Pt0.3Pd0.3/C, (f) Co0.5Pt0.25Pd0.25/C, (g) Co0.7Pt0.15Pd0.15/C, and (h) Co0.9Pt0.05Pd0.05/C. (B) CVs of formic acid electro-oxidation on Pt0.35Pd0.35Co0.30/C, Pt0.65Co0.35/C, Pd0.65Co0.35/C, Pd/C and Pt/C respectively in 0.5 mol L-1H2SO4+ 0.5 mol L-1HCOOH solutions. (C) CAs for Pt0.35Pd0.35Co0.30/C, Pd0.65Co0.35/C, and Pd/C was recorded at 0.35 V for 3000 s | |
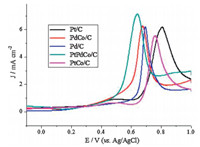
Fig. 7 displays the CO stripping voltammograms of Pt0.35Pd0.35Co0.30/C,Pt0.65Co0.35/C,Pd0.65Co0.35/C and Pd/C and Pt/ C. Prior to CO adsorption,the electrolytes were deoxygenated by bubbling with N2 for 30 min. For CO stripping experiments,CO was adsorbed on the pre-cleaned electrode by holding the potential at -0.1 V for 5 min by bubbling CO gas through the electrolyte solution. CO dissolved in the electrolyte solution was then removed by purging nitrogen through the solution for 15 min. For Pt0.35Pd0.35Co0.30/C catalyst,a sharp peak was observed at around 0.63 V. Compared to CO stripping voltammogram of Pt0.65Co0.35/C,Pd0.65Co0.35/C,Pt/C and Pd/C catalysts presented a sharp peak at a potential of 0.76,0.68,0.80 and 0.69 V,suggesting a higher CO oxidation activity on Pt0.35Pd0.35Co0.30/C catalysts because of the more negative potential. What is more,the onset potential of Pt0.35Pd0.35Co0.30/C and Pd0.65Co0.35/C were more negative than that of catalysts,which indicated Pt0.35Pd0.35Co0.30/C show higher CO tolerance.
|
Download:
|
| Fig. 7. CO stripping curves of the Pt0.35Pd0.35Co0.30/C, Pt0.65Co0.35/C, Pd0.65Co0.35/C, Pt/C and Pd/C in 0.5 mol L-1H2SO4solution. | |
In summary,the Pt0.35Pd0.35Co0.30 nanoalloy supported on carbon has been successfully applied as a stable catalyst for methanol,formic acid electro-oxidation and CO stripping experiments. Compared with Pt0.65Co0.35/C,Pd0.65Co0.35/C,Pt/C and Pd/C catalyst,Pt0.35Pd0.35Co0.30/C exhibited relatively high durability and strong poisoning resistance,and the Pt-mass activity was 3.6 times higher than that of Pt/C in methanol oxidation reaction. Meanwhile,the Pt0.35Pd0.35Co0.30/C exhibited excellent activity with higher current density and higher CO tolerance than that of Pt/C in formic acid electro-oxidation. The enhanced catalytic performance of Pt0.35Pd0.35Co0.30/C may be attributed to its special composition and surface electronic state. This implies that the Pt0.35Pd0.35Co0.30/C catalysts would become alternatives to commercial Pt/C in fuel cells. Acknowledgments
This work was supported by NSFC (No. 21373116),Tianjin Natural Science Research Fund (No. 13JCYBJC18300),RFDP (No. 20120031110005) and MOE Innovation Team of China (No. IRT13022).
| [1] | S. Strnivasan, R. Mosdale, P. Stevens, C. Yang, Fuel cells: reaching the era of clean and efficient power generation in the twenty-first century, Ann. Rev. Energy Environ. 24 (1999) 281-328. |
| [2] | S.J. Guo, S. Zhang, S.H. Sun, Tuning nanoparticle catalysis for the oxygen reduction reaction, Angew. Chem. Int. Ed. 52 (2013) 8526-8544. |
| [3] | S. Strnivasan, Fuel Cells: From Fundamentals to Applications, Springer, New York, 2006. |
| [4] | Y.H. Bing, H.S. Liu, L. Zhang, D. Ghosh, J.J. Zhang, Nanostructured Pt-alloy electrocatalysts for PEM fuel cell oxygen reduction reaction, Chem. Soc. Rev. 39 (2010) 2184-2201. |
| [5] | S.J. Yoo, T.Y. Jeon, K.S. Kim, T.H. Lim, Y.E. Sung, Multilayered Pt/Ru nanorods with controllable bimetallic sites as methanol oxidation catalysts, Phys. Chem. Chem. Phys. 12 (2010) 15240-15246. |
| [6] | S.Y. Shen, T.S. Zhao, J.B. Xu, Y.S. Li, Synthesis of PdNi catalysts for the oxidation of ethanol in alkaline direct ethanol fuel cells, J. Power Sources 195 (2010) 1001-1006. |
| [7] | J. Kugai, T. Moriya, S. Seino, et al., CeO2-supported Pt-Cu alloy nanoparticles synthesized by radiolytic process for highly selective CO oxidation, Int. J. Hydrogen Energy 37 (2012) 4787-4797. |
| [8] | G.A. Camara, R.B. De Lima, T. lwasita, The influence of PtRu atomic composition on the yields of ethanol oxidation: a study by in situ FTIR spectroscopy, J. Electroanal. Chem. 585 (2005) 128-131. |
| [9] | M. Nie, H.L. Tang, Z. Wei, S.P. Jiang, P.K. Shen, Highly efficient AuPd-WC/C electrocatalyst for ethanol oxidation, Electrochem. Commun. 9 (2007) 2375-2379. |
| [10] | E. Antolini, F. Colmati, E.R. Gonzalez, Ethanol oxidation on carbon supported (PtSn)alloy/SnO2 and (PtSnPd)alloy/SnO2 catalysts with a fixed Pt/SnO2 atomic ratio: effect of the alloy phase characteristics, J. Power Sources 193 (2009) 555-561. |
| [11] | E. Lee, I.S. Park, A. Manthiram, Synthesis and characterization of Pt-Sn-Pd/C catalysts for ethanol electro-oxidation reaction, J. Phys. Chem. C 114 (2010) 10634-10640. |
| [12] | J. Datta, A. Dutta, S. Mukherjee, The beneficial role of the cometals Pd and Au in the carbon-supported PtPdAu catalyst toward promoting ethanol oxidation kinetics in alkaline fuel cells: temperature effect and reaction mechanism, J. Phys. Chem. C 115 (2011) 15324-15334. |
| [13] | M. Watanabe, K. Tsurumi, T. Nakamura, T. Nakamura, P. Stonehart, Activity and stability of ordered and disordered Co-Pt alloys for phosphoric acid fuel cells, J. Electrochem. Soc. 141 (1994) 2659-2668. |
| [14] | E. Antolini, J.R.C. Salaado, E.R. Gonzalez, The stability of Pt-M (M=first row transition metal) alloy catalysts and its effect on the activity in low temperature fuel cells: a literature review and tests on a Pt-Co catalyst, J. Power Sources 160 (2006) 957-968. |
| [15] | V. Mazumder, M. Chi, M.N. Mankin, et al., A facile synthesis of MPd (M=Co, Cu) nanoparticles and their catalysis for formic acid oxidation, Nano Lett. 12 (2012) 1102-1106. |
| [16] | S.K. Singh, Q. Xu, Complete conversion of hydrous hydrazine to hydrogen at room temperature for chemical hydrogen storage, J. Am. Chem. Soc. 131 (2009) 18032-18033. |
| [17] | D. Sun, V. Mazumder, O. Metin, S.H. Sun, Catalytic hydrolysis of ammonia borane via cobalt palladium nanoparticles, ACS Nano 5 (2011) 6458-6464. |
| [18] | C. Wang, M. Chi, D. Li, et al., Synthesis of homogeneous Pt-bimetallic nanoparticles as highly efficient electrocatalysts, ACS Catal. 1 (2011) 1355-1359. |
| [19] | E. Bertin, S. Garbarino, A. Ponrouch, D. Guay, Synthesis and characterization of PtCo nanowires for the electro-oxidation of methanol, J. Power Sources 206 (2012) 20-28. |
| [20] | B.M. Luo, X.B. Yan, S. Xu, Q.J. Xue, synthesis of worμ-like PtCo nanotubes for methanol oxidation, Electrochem. Commun. 30 (2013) 71-74. |
| [21] | H. Zhao, L. Pan, J. Jin, L. Li, J. Xu, PtCo/polypyrrole-multiwalled carbon nanotube complex cathode catalyst containing two types of oxygen reduction active sites used in direct methanol fuel cells, Fuel Cell 12 (2012) 876-882. |
| [22] | H.J. Huang, Y. Fan, X. Wang, Low-defect multi-walled carbon nanotubes supported PtCo alloy nanoparticles with remarkable performance for electrooxidation of methanol, Electrochim. Acta 80 (2012) 118-125. |
| [23] | O.N. Senkov, D.B. Miracle, Effect of the atomic size distribution on glass forming ability of amorphous metallic alloys, Mater. Res. Bull. 36 (2001) 2183-2198. |
| [24] | A.P. Tsai, A test of Hume-Rothery rules for stable quasicrystals, J. Non-Cryst. Solids 334 (2004) 317-322. |
| [25] | J.W. Hong, D. Kim, Y.W. Lee, et al., Atomic-distribution-dependent electrocatalytic activity of Au-Pd bimetallic nanocrystals, Angew. Chem. Int. Ed. 50 (2011) 8876-8880. |
| [26] | G. Giovannetti, P.A. Khomyakov, G. Brocks, et al., Doping graphene with metal contacts, Phys. Rev. Lett. 101 (2008) 026803(1)-026803(4). |
| [27] | R. Larsen, S. Ha, J. Zakzeski, R.I. Masel, Unusually active palladiuμ-based catalysts for the electrooxidation of formic acid, J. Power Sources 157 (2006) 78-84. |
| [28] | Y. Lu, W. Chen, Nanoneedle-covered Pd-Ag nanotubes: high electrocatalytic activity for formic acid oxidation, J. Phys. Chem. C 114 (2010) 21190-21200. |




