Due to high power density,long cycle life,excellent pulse charge/discharge capability,etc. supercapacitors offer a promising prospect to meet increasing energy storage requirements [1]. Activated carbons (ACs) have been considered one of the most promising candidates for supercapacitor electrodes because of their high surface areas,low cost,and commercial availability [2]. However,the main pores of ACs are micropores,leading to poor accessibility of electrolyte into carbon surfaces. Recently,it has been reported that mesopores are favorable for high transportation speed of electrolyte ions,and macropores promote the formation of ion buffering areas that minimizes the transportation distance of the ions [3]. Micropores offer plentiful adsorbing sites for ions and enhance the electrochemical capacitance [2, 4]. Therefore,it is desirable to design porous carbons with multi-scale pores to be used in supercapacitors. An optimum choice would be to fabricate porous carbons with pore channels interconnected with large pores,which enables high surface area and efficient ion diffusion pathways. Recently,there have been many efforts devoted to the synthesis of hierarchical porous carbons (HPCs) with ordered or aperiodic pore structure to achieve a better capacitive feature for supercapacitors [3c,5].
Template synthesis is a widespread approach used for HPCs [6], which generally includes two steps: to form an ordered packing structure and then to penetrate carbon sources into the voids to solidify the porous structure. Removal of colloids leads to a 3D macropore array and macro or/and mesopore windows due to the overlapping of the neighboring pores. For example,Yi et al. reported the synthesis of HPCs with macropores (170 nm) and mesopores (20-30 nm) by using polystyrene spheres as the hard template [6a]. Denget al. fabricated HPCs with macropore cores (230-430 nm),pore windows (30-65 nm) and mesopores (~11 nm) using SiO2 colloids and F127 as hard/soft templates [6c]. The procedure involves close-packing of the silica colloids, formation of mesostructure in the voids by solvent evaporationinduced self-assembly (EISA),thermosetting-carbonization of the carbon source,and template removal. Such templated carbons show highly ordered and well defined porous structure,however, the use of a colloid package to create a 3D macroporous array and the use of surfactant viaEISA to fabricate ordered mesopores is difficult and unfeasible,especially from the industrial point of view.
Emulsions are a member of the colloid family which consist of an emulsifier and at least two immiscible or slightly miscible phases [7]. Emulsion polymerization is usually adopted for manufacturing some commercially important polymers such as poly(vinyl chloride) [8]. Herein,we demonstrated a novel synthesis of HPCs based on nanosilica-embedded oil-in-water (O/W) emulsion-templated polymerization. The removal of the oil phase enveloped by the aqueous continuous phase in the polymeric emulsion generates macropore cores,and the removal of silica colloids generates meso/macropore windows. Further activation of such carbons by KOH brings micropores and high specific surface area. Our methodology overcomes the disadvantages of pre-packing the 3D crystal array and is supposed to be suitable for the widespread application of HPCs as electrode materials in supercapacitors due to their charge/discharge capability under large current density coupling with a reasonable specific capacitance. 2. Experimental
Liquid paraffin (ρ= 0.84 g/cm3),Span 80,Tween 80,resorcinol, formaldehyde (37-40 wt%),KOH,hydrochloric acid (36-38 wt%), hydrofluoric acid (49 wt%),and ethanol were analytical reagents and were purchased from Sinopharm Chemical Reagent Co.,Ltd. Colloidal silica sol (30 wt%) was purchased from Zhejiang Yuda Chemical Industry Co.,Ltd. Graphite was obtained from Shanghai Colloid Chemical Plant. Nickel foil was manufactured by Shanghai Hongxiang Plant. Polytetrafluoroethylene (PTFE) was purchased from Shanghai 3F New Materials Co.,Ltd. Pure N2 was supplied by Shanghai BOC Special Gases Sales Service Co.,Ltd. All materials were used as received without further purification. Water used was distilled water.
Liquid paraffin (6.7 mL),which serves as an oil phase,was placed in a flask with a thermostat of 40°C. Span 80 (1.2 g) and Tween 80 (1.6 g) were added into the flask. Silica sol (4.5 g),resorcinol (4.4 g), and formaldehyde (6.5 g) were mixed with water (4.2 mL) to obtain an aqueous phase. The aqueous phase was added into the flask containing oil phase and emulsifiers under stirring to prepare an O/Wemulsion. Then,HCl solution (2 mol/L) was added into the flask, and resorcinol and formaldehyde (R/F) began to polymerize. The polymerized emulsion was washed by water,followed by drying at 100°C and carbonization at 850°CinaN2 flow to obtain silica/ carbon composite. Meso/macroporous carbons (MMCs) were prepared by the etching of silica from the composite using HF solution. Saturated KOH solution was mixed with such carbons at KOH/carbon mass ratios of 3-7:1 for 2 h. The samples were dried and activated at 850°C to fabricate HPCs (denoted as HPC-xwherex denotes the mass ratios of KOH to carbon).
Scanning electron microscopy (SEM) observation was conducted on a Hitachi S-4800 apparatus. N2 adsorption and desorption analysis was obtained at -196°C by using a Micromeritics Tristar 3000 gas adsorption analyzer. The specific surface area was measured by using the Brunauer-Emmett-Teller (BET) method,the pore size distribution was calculated from the adsorption branches of isotherms by using Barett-Joyner-Halenda (BJH) model,and the total pore volume was estimated from the adsorbed amount at a relative pressureP/P0of 0.97. Electrochemical measurement was done by using a typical three-electrode system in which saturated calomel electrode (SCE) was used as a reference electrode and nickel foam as a counter electrode. The working electrode was prepared as follows: HPCs (80 wt%), graphite (10 wt%) and polytetrafluoroethylene (10 wt%) were mixed with ethanol to form a paste. The past was pressed between two pieces of nickel foam under 30 MPa,and then 1.0 mm thick electrode was dried overnight at 100°C. Cyclic voltammogram (CV) and galvanostatic charge/discharge (GCD) measurements were carried out in 6.0 mol/L KOH electrolyte solution using a CHI660D electrochemical workstation,and the potential window was chosen in the range of -1.0 V to 0 Vvs. SCE. The specific capacitances of HPCs were calculated from GCD datum by using the method described in Ref. [3c]. 3. Results and discussion
Fig. 1 shows SEM images of MMCs which show a 3D open-cell structure with macropore cores (P1) interconnected by meso/ macropore windows (P2). The sizes of macropore cores are several hundreds of nanometers,which resulted from the removal of oil droplets capsulated by R/F polymer framework. Emulsion-templated polymerization generally leads to micrometer-scaled macropores [9]. Silica colloids are considered emulsion stabilizers because they could create a solid protective barrier at the liquid/ liquid interface [10],that is,nanosilica introduced into the aqueous phase prevents the coalescence of emulsion droplets to form bigger ones. As a result,the droplets of liquid paraffin in nanosilicastabilized emulsion are small; thereafter,polymerization and carbonization of the O/W emulsion brings submicrometer macropores (Fig. 1A). Furthermore,silica colloids form aggregations in aqueous phase due to abundant -OH on their surfaces [11]. There are also H-bond interactions between these -OH and those located on the head of emulsifiers,which attracted the aggregations closely to the oil/water interface. Removal of the aggregations by HF leads to open meso/macropore windows (Fig. 1B). In addition, the carbons have thin walls; when such carbon electrodes are immersed in the electrolyte,the macroporous cores could form ion-buffering reservoirs,and the thin walls around them are covered by it,thus giving a very short diffusion distance.

|
Download:
|
| Fig. 1. Low (A) and higher (B) magnification SEM images of MMCs (P1: macropore cores and P2: open meso/macropore windows). | |
Fig. 2 shows N2 adsorption and desorption isotherms of HPCs. The isotherms show a type IV curve with an obvious hysteresis loop at P/P0 of 0.4-1.0,which is a typical feature of capillary condensation that takes place in mesopores. Silica colloids used have a uniform size of about 10 nm [6e]. However,HPCs do not show a pore size distribution corresponding to that of nanosilica, but exhibit a relatively broad pore size ranging from 10 nm to 100 nm (calculated from the adsorption isotherms by BJH model). As discussed above,silica colloids easily aggregate in aqueous solution due to their surface hydroxyl groups and the subsequent H-bonding interaction. Thus,it is the silica aggregation instead of isolated nanosilica particle that plays the role of the template. This result also matches that of SEM observation. Table 1 shows the porous textures of HPCs which have surface areas of 1086- 1501 m2/g,total pore volumes of 0.47-0.91 cm3/g calculated at P/P0of 0.97 [12],and micropore volumes of 0.26-0.66 cm3/g. When increasing the KOH/carbon mass ratio from 3:1 to 5:1,the above parameters of HPCs increased due to the interior etching caused by KOH [13],but decreased when the KOH/carbon mass ratio reaches 6:1 or above due to the over reaction of KOH with the carbons.

|
Download:
|
| Fig. 2. Nitrogen adsorption and desorption isotherms of HPCs. | |
| Table 1 Porous textures of HPCs. |
Fig. 3 shows the schematic synthesis of HPCs by nanosilicaembedded emulsion-templated polymerization. When mixing the aqueous phase with oil phase and emulsifiers,a stable O/W emulsion with nanosilica aggregation,R/F and water in the external phase,and liquid paraffin in the internal phase were obtained. Liquid paraffin was enveloped by the aqueous phase, while the amphiphiles closely adsorbed at the oil/water interfaces with their hydrophilic heads moving to the water phase and the hydrophobic tails orienting to the oil phase. R/F polymerization offers a polymer framework. The removal of liquid paraffin by carbonization generates macropore cores,and the etching of silica aggregations brings meso/macropore windows. Also,abundant micropores were created by KOH activation. Macropore cores offer ion-buffering reservoirs for decreasing electrolyte diffusion distance; meso/macropores windows supply low-resistant channels for the ions,while the micropores offer adsorbing sites for the ions and strengthen electric double-layer capacitance.
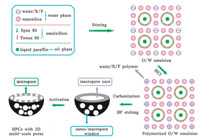
|
Download:
|
| Fig. 3. Schematic synthesis of HPCs with 3D multi-scale pores based on nanosilica-embedded O/W emulsion-templated polymerization. | |
Fig. 4A shows CV curves of MMCs electrodes at different scanning rates in 6.0 mol/L KOH aqueous solution. These CV curves have quasi-rectangular shapes at scanning rates ranging from 5 mV/s to 50 mV/s,indicating good capacitance behavior. However,the CV curve of MMCs veered away from the rectangle-shape at a scanning rate of 100 mV/s,which suggests a resistance-like electrochemical behavior. MMCs were further activated by KOH to obtain HPCs with new-generated micropores and higher BET surface areas. Fig. 4B exhibits CV curves of the HPC-5 electrode under different scanning rates in 6.0 mol/L KOH aqueous solution. These CV curves were characterized with nearly rectangle shapes between 5 mV/s and 200 mV/s,which imply that an ideal capacitance behavior occurred at supercapacitor electrodes with quick charge/discharge feature [14]. By comparison with HPCs,the CV curve for MMCs electrode material becomes somewhat tilted under a scanning rate of 100 mV/s. Besides,HPC-5 shows bigger inner integrated areas than that of MMCs electrode under the same scanning rate. The capacitance of a material is in proportion to the integrated area of its CV curve,and thus the specific capacitances of HPC-5 are much larger than that of MMCs. The improvement of CV performances of HPCs should be ascribed to their new-generated micropores and the consequent higher specific surface areas which could offer more adsorbing sites for electrolyte ions. Besides,HPC-5 electrode has the largest inner integrated areas calculated from its CV curves under wide scanning rates compared with other HPCs. The relatively high BET areas and large mean pore size contribute to the good electrochemical properties of HPC-5 electrode. HPC-5 has somewhat smaller BET surface area than that of HPC-4 (1501 m2/g),but it has an average pore size of 2.7 nm,larger than that of HPC-4 (2.4 nm). Bigger pores are favorable for accessibility of electrolyte solution throughout the electrode materials and consequently fast ion diffusion or transportation required for supercapacitors. As a result,HPC-5 electrode exhibits an improved electrochemical performance. This result is similar with our previous work in which mesoporous carbon microspheres with BET surface area of 1010 m2/g and average pore size of 4.0 nm show much better electrochemical performances compared with the sample that has the above two parameters of 1212 m2/g and 3.6 nm,respectively [15].

|
Download:
|
| Fig. 4. CV curves of MMCs (A) and HPC-5 (B) electrodes in 6.0 mol/L KOH solution under different scanning rates. | |
Fig. 5 shows GCD curves of HPC-5 electrode in KOH aqueous solution under different current densities. The GCD curves of HPC-5 between 0 and -1.0 V vs. SCE at loading current densities of 1.0-10.0 A/g exhibit regularly triangular shapes. This result reveals that the capacitor is provided by electric double layer charging/ discharging with good stability. Besides,the GCD curve of HPC-5 under a very high loading current density of 30.0 A/g still retains a typical triangle-shape. HPC-5 has a specific capacitance of 172 F/g at 1.0 A/g,and 119 F/g at 30.0 A/g. One of the challenges to the practical application of supercapacitors is to prepare high performance electrodes with rapid charge/discharge capability associated with a fair capacitance usage [16]. However,micro-,or mesoporous carbons which have a single pore size face much difficulty to achieve such features. Microporous carbons encounter a low current density in charge and discharge process although they exhibit large specific capacitance. Templated ordered mesoporous carbons provide reasonable capability of charge/ discharge under large current density,but suffer low specific capacitance due to insufficient surface areas compared with microporous carbons [5c,17]. For example,mesoporous carbon CMK-3 prepared by a nanocasting method using ordered mesoporous silica SBA-15 as a template has a capacitance below 100 F/g [17b]. Therefore,the above-mentioned shortages associated with common porous carbons impede their widespread applications for supercapacitors. HPCs presented here combine high surface area (~1500 m2/g) and well-developed,multi-scaled, and 3D open-cell structures,and thus show the charge/discharge capability under 30 A/g coupling with a reasonable electrochemical capacitance (119 F/g). Therefore,the HPCs are potentially important for application in electrode materials which would meet the requirement of charge/discharge under high current density in supercapacitors.
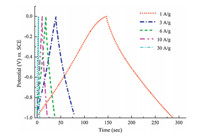
|
Download:
|
| Fig. 5. GCD curves of HPC-5 electrode in 6.0 mol/L KOH solution under different loading current densities. | |
In conclusion,HPCs were fabricated based on a novel nanosilica-embedded O/W emulsion-templated polymerization. HPCs show high surface areas and well-developed 3D open-cell structure combining macropore cores,meso/macropore windows and micropores. A typical sample as a supercapacitor electrode shows a specific capacitance of 172 F/g at 1.0 A/g,and 119 F/g even at 30.0 A/g in 6.0 mol/L KOH solution. Our methodology is supposed to be suitable for the widespread application of HPCs as electrode materials in supercapacitors due to the charge/ discharge capability under large current density coupling with a reasonable specific capacitance. Besides,we also believe that the present approach could be extended to other well-chosen emulsion systems as well as colloids with various dimensions and properties to design and synthesize HPCs. Acknowledgments
The project was supported by the National Natural Science Foundation of China (Nos. 21207099 and 21273162),Science and Technology Commission of Shanghai Municipality,China (Nos. 11nm0501000 and 12ZR1451100),and Key Subject of Shanghai Municipal Education Commission (No. J50102).
| [1] | (a) H. Jiang, P.S. Lee, C.Z. Li, 3D carbon based nanostructures for advanced supercapacitors, Energy Environ. Sci. 6 (2013) 41-53;(b) H. Jiang, J. Ma, C.Z. Li, Mesoporous carbon incorporated metal oxide nanomaterials as supercapacitor electrodes, Adv. Mater. 24 (2012) 4197-4202;(c) Y.G. Wang, H.Q. Li, Y.Y. Xia, Ordered whiskerlike polyaniline grown on the surface of mesoporous carbon and its electrochemical capacitance performance, Adv. Mater. 18 (2006) 2619-2623;(d) J.S. Qian, M.X. Liu, L.H. Gan, et al., A seeded synthetic strategy for uniform polymer and carbon nanospheres with tunable sizes for high performance electrochemical energy storage, Chem. Commun. 49 (2013) 3043-3045;(e) M.X. Liu, L.H. Gan, W. Xiong, et al., Nickel-doped activated mesoporous carbon microspheres with partially graphitic structure for supercapacitors, Energy Fuels 27 (2013) 1168-1173;(f) L.R. Wang, F. Ran, Y.T. Tan, et al., Coral reef-like polyanaline nanotubes prepared by a reactive template of manganese oxide for supercapacitor electrode, Chin. Chem. Lett. 22 (2011) 964-968. |
| [2] | D.Y. Qu, Studies of the activated carbons used in double-layer supercapacitors, J. Power Sources 109 (2002) 403-411. |
| [3] | (a) M.X. Liu, L.H. Gan, W. Xiong, et al., Partially graphitic micro-and mesoporous carbon microspheres for supercapacitors, Chin. Chem. Lett. 24 (2013) 1027-1040;(b) G. Hasegawa, M. Aoki, K. Kanamori, et al., Monolithic electrode for electric double-layer capacitors based on macro/meso/microporous S-containing activated carbon with high surface area, J. Mater. Chem. 21 (2011) 2060-2063;(c) Y.K. Lü, L.H. Gan, M.X. Liu, et al., A self-template synthesis of hierarchical porous carbon foams based on banana peel for supercapacitor electrodes, J. Power Sources 209 (2012) 152-157. |
| [4] | D.Y. Qu, H. Shi, Studies of activated carbons used in double-layer capacitors, J. Power Sources 74 (1998) 99-107. |
| [5] | (a) D.W. Wang, F. Li, M. Liu, G.Q. Lu, H.M. Cheng, 3D aperiodic hierarchical porous graphitic carbon material for high-rate electrochemical capacitive energy storage, Angew. Chem. Int. Ed. 47 (2008) 373-376;(b) Z.Y. Wang, E.R. Kiesel, A. Stein, Silica-free syntheses of hierarchically ordered macroporous polymer and carbon monoliths with controllable mesoporosity, J. Mater. Chem. 18 (2008) 2194-2200;(c) W. Xing, C.C. Huang, S.P. Zhuo, et al., Hierarchical porous carbons with high performance for supercapacitor electrodes, Carbon 47 (2009) 1715-1722;(d) Q. Li, R.R. Jiang, Y.Q. Dou, et al., Synthesis of mesoporous carbon spheres with a hierarchical pore structure for the electrochemical double-layer capacitor, Carbon 49 (2011) 1248-1257. |
| [6] | (a) J. Yi, X.P. Li, S.J. Hua, et al., Preparation of hierarchical porous carbon and its rate performance as anode of lithium ion battery, J. Power Sources 196 (2011) 6670-6675;(b) S.W. Woo, K. Dokko, H. Nakano, K. Kanamura, Preparation of three dimensionally ordered macroporous carbon with mesoporous walls for electric doublelayer capacitors, J. Mater. Chem. 18 (2008) 1674-1680;(c) Y.H. Deng, C. Liu, T. Yu, et al., Facile synthesis of hierarchically porous carbons from dual colloidal crystal/block copolymer template approach, Chem. Mater. 19 (2007) 3271-3277;(d) H. Yamada, H. Nakamura, F. Nakahara, I. Moriguchi, T. Kudo, Electrochemical study of high electrochemical double layer capacitance of ordered porous carbons with both meso/macropores and micropores, J. Phys. Chem. C 111 (2007) 227-233;(e) M.X. Liu, L.H. Gan, C. Tian, et al., Dual template approach for the synthesis of hierarchically mesocellular carbon foams, Chin. Chem. Lett. 20 (2009) 123-126. |
| [7] | (a) L.F. Chen, Y.Z. Shang, H.L. Liu, Y. Hu, Effect of the spacer group of cationic Gemini surfactant on microemulsion phase behavior, J. Colloid Interface Sci. 301 (2006) 644-650;(b) A. Menner, R. Powell, A. Bismarck, Open porous polymer foams via inverse emulsion polymerization: should the definition of high internal phase (ratio) emulsions be extended? Macromolecules 39 (2006) 2034-2035. |
| [8] | P.V. Smallwood, The formation of grains of suspension poly(vinyl chloride), Polymer 27 (1986) 1609-1618. |
| [9] | (a) V.O. Ikem, A. Menner, A. Bismarck, High-porosity macroporous polymers sythesized from titania-particle-stabilized medium and high internal phase emulsions, Langmuir 26 (2010) 8836-8841;(b) M.X. Liu, L.H. Gan, F.Q. Zhao, et al., Carbon foams prepared by an oil-in-water emulsion method, Carbon 45 (2007) 2710-2712;(c) M.X. Liu, L.H. Gan, Z.J. Xu, et al., Unusual phase inversion behavior in an emulsion polymerization system caused by ammonia, Chem. Lett. 39 (2010) 274-275. |
| [10] | B.P. Binks, S.O. Lumsdon, Stability of oil-in-water emulsions stabilised by silica particles, Phys. Chem. Chem. Phys. 1 (1999) 3007-3016. |
| [11] | S.J. Han, T. Hyeon, Simple silica-particle template synthesis of mesoporous carbons, Chem. Commun. (19) (1999) 1955-1956. |
| [12] | M.A. Springuel-Huet, J.L. Bonardet, A. Gédéon, et al., Mechanical properties of mesoporous silicas and alumina-silicas MCM-41 and SBA-15 studied by N2 adsorption and 129Xe NMR, Microporous Mesoporous Mater. 44-45 (2001) 775-784. |
| [13] | F.C. Wu, R.L. Tseng, C.C. Hu, C.C. Wang, Physical and electrochemical characterization of activated carbons prepared from firwoods for supercapacitors, J. Power Sources 138 (2004) 351-359. |
| [14] | P. Simon, Y. Gogotsi, Materials for electrochemical capacitors, Nat. Mater. 7 (2008) 845-854. |
| [15] | W. Xiong, M.X. Liu, L. Gan, et al., A novel synthesis of mesoporous carbon microspheres for supercapacitor electrodes, J. Power Sources 196 (2011) 10461-10464. |
| [16] | L.M. Dai, D.W. Chang, J.B. Baek, W. Lu, Carbon nanomaterials for advanced energy conversion and storage, Small 8 (2012) 1130-1366. |
| [17] | (a) D.W. Wang, F. Li, Z.G. Chen, G.Q. Lu, H.M. Cheng, Synthesis and electrochemical property of boron-doped mesoporous carbon in supercapacitor, Chem. Mater. 20 (2008) 7195-7200;(b) H.S. Zhou, S.M. Zhu, M. Hibino, I. Honma, Electrochemical capacitance of selfordered mesoporous carbon, J. Power Sources 122 (2003) 219-223;(c) N. Brun, S.R.S. Prabaharan, M. Morcrette, et al., Hard macrocellular silica Si(HIPE) foams templating micro/macroporous carbonaceous monoliths: applications as lithium ion battery negative electrodes and electrochemical capacitors, Adv. Funct. Mater. 19 (2009) 3136-3145. |





