LiCoO2 has been widely used as commercial cathode materials for lithium ion batteries,but still has some limitations due to its relatively high cost and the toxicity of cobalt. Research and development of new alternative cathode materials for lithium ion batteries are ongoing,such as layered LiNiO2,LiMniO2 and spinel LiMn2O4 [1, 2, 3, 4]. Unfortunately,these materials still have significant drawbacks. LiNi1-x-yCoxMnyO2 series,which integrate the features of LiCoO2,LiNiO2 and LiMniO2,have attracted much attention for their high capacity,low cost,good cycling stability and safety. It is well known that Ni provides high capacity but poor thermal stability,while Mn maintains excellent cycling performance and safety. And Co offers increased electronic conductivity resulting in an excellent rate capability. In most cases,only Ni and Co are electroactive,whereas Mn has the nominal oxidation state of +4 and is electrochemically inert. Hence,the intrinsic properties of LiNi1-x-yCoxMnyO2 materials strongly depend on the amount of the metal ions (Ni,Co,Mn) [5, 6, 7].
Concentration-gradient structured materials have been studied as cathode material for lithium ion batteries. Sun et al. prepared a LiNi0.75Co0.10Mn0.1502 cathode material where the nickel concentration decreases linearly whereas the manganese concentration increases linearly from the centre to the outer layer of each particle. Huang et al. synthesized spherical LiNixCo1-2xMnxO2 (x = 0.333,0.4,0.416,0.45) precursors with Co concentrationgradient. Koenig et al. prepared a Li1.2(Mn0.62Ni0.38)0.802 material, with the transition metal composition at the surface and interior of a particle formed with a continuous gradient in concentration from Ni-enriched centre to Mn-enriched surface [8, 9, 10]. In this paper, the LiNi0.6Co0.2Mn0.2O2 material is chosen as the study object. Various methods are applied to synthesize LiNi0.6Co0.2Mn0.2O2 cathode materials,such as the hydroxide co-precipitation method [11, 12, 13, 14, 15, 16],the carbonate co-precipitation method [17, 18],the combustion method [19],the solid-state method [6, 20, 21, 22] and the spray-drying method [20, 22, 23]. In this research,the Ni0.6Co0.2Mn0.2(OH)2 precursor is prepared by a hydroxide coprecipitated method. In this material,the concentration of nickel decreases gradually from the centre towards the outer layer of the particle,whereas the concentration of manganese increases gradually so that the manganese-rich and nickel-poor outer layer can stabilize the material,especially during high-voltage cycling.
2. ExperimentalNiSO4·6H2O,CoSO4·7H2O and MnSO4·H2O were used as starting materials. The nickel-rich solution of NiSO4·6H2O and CoSO4·7H2O (Ni:Co = 0.85:0.15,molar ratio) was named solution A. The manganese-rich aqueous solution of NiSO4·6H2O,CoSO4·7H2O and MnSO4·H2O (Ni:Co:Mn = 1:3:6,molar ratio) was named solution B. Solution A was pumped into solution B in flow rate V. simultaneously,the mixed solution of A and B was agitated vigorously and slowly pumped into a continuous stirred tank reactor (capacity 10 L) in flow rate 2 V. The reactor was under nitrogen atmosphere. The volume of solutions A and B was equal and the two solutions were exhausted at the same time. This protocol allowed the concentration of Ni and Mn fed into the reactor to change continuously,but in opposite direction. At the same time,a 4 mol/L NaOH solution (aq) and an appropriate amount of NH4OH solution (aq) as a chelating agent were also separately dropped into the reactor. After reacting for a certain time,the precipitate was filtered and washed for several times to remove the residual ions (Na+,SO4 2- and other impurity ions). The precipitate was then dried at 80 ℃ overnight. The concentration of the solution,pH value,amount of NH4OH,stirring speed and temperature in the reactor must be carefully controlled. The obtained spherical precursor and 5% excess LiOH·H2O powders were mixed thoroughly. The mixture was first calcined at 550 ℃ for 4 h in air,and then heated at 820 ℃ for 12 h in flowing oxygen to obtain the LiNi0.6Co0.2Mn0.2O2 powders. The synthesized compounds were characterized by XRD (Riguku u/u diffractometer with Cu Ka radiation(r = 1.54056Å ). XRD data were obtained at 2u = 10-808,with a scan speed of 28/min. The particle morphology of the powders was observed using a scanning electron microscopy equipped with an EDXS energy disperse X-ray spectrometer (SEM, Hitachi X-650). The chemical composition of the resulting compounds was analyzed by atomic absorption spectroscopy (AA spectrophotometer,Zhimadu 6650). The charge/discharge tests were carried out by assembling 2025-type coin cells with a lithium metal anode,working cathode and Celgard 2400 microporous membrane. The working cathode was fabricated by 80 wt% LiNi0.6Co0.2Mn0.2O2,10 wt% acetylene black and 10 wt% PVDF binder. A 1 mol/Lsolution of LiPF6 dissolved in ethylene carbonate (EC)/dimethyl carbonate(DMC) (1:1,v/v) was employed as the electrolyte. The charge-discharge tests were set to be charged and discharged between 2.8 and 4.3 V at 0.1 C rate in the first 2 cycles and then at 1 C rate in the following 100 cycles at 25 ℃ or 60 ℃. Cyclic voltammograms were measured by a Solartron 1287 electrochemical interface between 2.8 and 4.5 V at a scan rate of 0.1 mV/s.
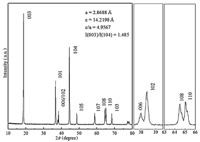
3. Results and discussionFig. 1 displays the XRD pattern of the Li[Ni0.6Co0.2Mn0.2]O2 material. The XRD pattern shows a clear split between the 006/012 and 108/110 peaks,which means that the materials have a better hexagonal structure. All diffraction peaks are indexed on the basis of the α-NaFeO2 structure (R3m) and no extra diffraction peaks from related secondary phases or impurities exist. Unit cell parameters are calculated and summarized in Fig. 1. The lattice parameters a and c are given as 2.8688 and 14.2198,which are similar to the values reported in the literature references [12- 14,16]. The intensity ratio of I(0 0 3)/I(1 0 4) is a sensitive parameter for determining the cation distribution in the lattice,where a value lower than 1.2 indicates a high degree of cation mixing,due primarily to the occupancy of other ions in the lithium region,and the higher the ratio is,the lower the level of cation mixing and the more beneficial to the lithium-ion transfer [22]. The intensity ratio I(0 0 3)/I(1 0 4) of this material is 1.485,which is larger than 1.2,implying that no obvious cation mixing is present in the LiNi0.6Co0.2Mn0.2O2 sample. In addition,the c/a ratio of this material is greater than 4.9,a value that generally represents a material with layered characteristics. Thus good electrochemical performance of LiNi0.6Co0.2Mn0.2O2 material can be expected.
|
Download:
|
| Fig. 1. XRD pattern of concentration-gradient LiNi0.6Co0.2Mn0.2O2. | |
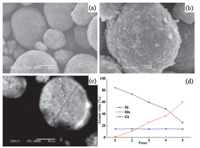
The SEM images of the concentration-gradient precursor and lithiated oxides are shown in Fig. 2(a) and (b). The morphology of the powders is nearly spherical and the estimated particle size is about 10 mm in diameter. Furthermore,the spherical morphology remains even after the high temperature calcinations. The crosssection of concentration-gradient precursor particles is also tested by EDXS. As shown in Fig. 2(c),the points collected in the EDXS spectra are labelled as 1,2,3,4 and 5 from outside. The concentration of Ni and Mn distribution tested by EDXS is shown in Fig. 2(d). It is obviously observed that the concentration of nickel decreases gradually from the centre towards the outer layer of the particle,whereas the concentration of manganese increases gradually,consistent with the experimental design. Atomic absorption spectroscopy is employed to measure the final elemental ratio in the sample. The atomic ratio of Li:Ni:Co:Mn in the LiNi0.6Co0.2Mn0.2O2 material is determined to be 1.03:0.602:0.196:0.202,which is nearly equal to the determined stoichiometric ratio.
|
Download:
|
| Fig. 2. SEM images of concentration-gradient Ni0.6Co0.2Mn0.2(OH)2 (a),LiNi0.6Co0.2Mn0.2O2 powders (b),the cross-section of precursor particle (c) and the concentration of Ni, Co and Mn in particle by EDXS testing (d). | |
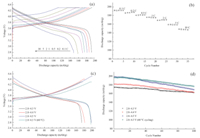
Fig. 3(a) exhibits the initial charge-discharge curves and rate cyclability of the LiNi0.6Co0.2Mn0.2O2 cells from 0.1 C to 10 C in the voltage of 2.8-4.3 V at 25 ℃. The cells are first cycled at 0.1 C (18 mA/g) and then at 0.2 C,0.5 C,1 C,2 C,5 C and 10 C for every five cycles. The cells deliver an initial discharge capacity of 191.5, 185.5,180.2,174.3,168.7,158.8 and 148.5 mAh/g from 0.1 C to 10 C,respectively. Excellent cycling performance can also be seen from Fig. 3(b). Even when discharged at 10 C,a capacity as high as 148.5 mAh/g is achieved,indicating that the LiNi0.6Co0.2Mn0.2O2 materials synthesized under the optimal conditions have superior rate capacities that have not been reported in the previous research [11, 12, 13, 14, 15, 16, 17, 18, 19, 20, 21, 22, 23]. Fig. 3(c) illustrates the charge-discharge profiles of assynthesized Li[Ni0.6Co0.2Mn0.2]O2 cathode at 1 C rate in the voltage range of 2.8-4.3,4.4 and 4.5 V,with the initial discharge capacities of 174.3,181.7 and 198.6 mAh/g,respectively. For elevated temperature test (60 ℃),the material delivers an initial discharge capacity of 196.9 mAh/g. It is very impressive that the first reversible discharge capacity at 1 C rate in the voltage range of 2.8- 4.3 V is as high as 174.3 mAh/g,which is higher than that of the similar solid solutions containing the same amount of Ni reported before [12, 13, 16]. The discharge capacity as a function of cycle number for the Li[Ni0.6Co0.2Mn0.2]O2 cathode cycled at 1 C rate in the voltage of 2.8-4.3,4.4 and 4.5 V is given in Fig. 3(d). In the voltage of 2.8-4.3 V,the discharge capacity gradually decreases from 174.3 mAh/g at the initial cycle to 162.3 mAh/g at the end of 100cycles,with a capacity retention of 93.1%. Between 2.8 and 4.4, 4.5 V,the discharge capacity decreases from 181.7,198.6 mAh/g to 162.9,169.2 mAh/g,respectively,after the same cycles. The corresponding capacity retentions are 89.6% and 85.2%,respectively. In the elevated temperature test (60 ℃),more than 89.7% of the initial discharge capacity is retained after 100 cycles. Therefore, the material exhibits the remarkable electrochemical performance especially the long cyclability,which is superior to the previously reported research results [12, 16]. The main reason for the outstanding cycle performance is largely attributed to the stable outer layer having lower Ni content and higher Mn content compared to core,which can reduce the cation mixing in the Nidepleted outer shell layer and dissolution of metal ions in the active material and stabilizes the surface structure of the oxide during the charge and discharge process [8].
|
Download:
|
| Fig. 3. The initial charge-discharge curves (a) and Rate cyclability (b) of LiNi0.6Co0.2Mn0.2O2 cells from 0.1 C to 10 C in the voltage of 2.8-4.3 V and The initial charge-discharge curves at a 1 C rate with raised upper cut-off voltage limit (c) and Cycling performance of LiNi0.6Co0.2Mn0.2O2 at 25 ℃ or 60 ℃ (d). | |
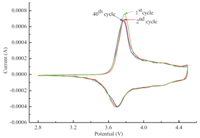
Cyclic voltammetry of the sample between 2.8 V and 4.5 V at a scan rate of 0.1 mV/s for the first,second and 40th cycle were carried out to investigate the structural stability of Li[Ni0.6Co0.2Mn0.2]O2 and the results are shown in Fig. 4. It can be seen that only one redox couple exhibits in the C-V curves, illustrating that no structural transitions exist from hexagonal to monoclinic in the voltage range of 2.8-4.5 V. It is clearly shown in Fig. 4 that the major anodic peak shifts from 3.781 V at the first cycle to 3.774 V and 3.768 V at the second and 40th cycle, respectively,while their corresponding redox reaction gaps are 82, 75 and 69 mV. However,there is no obvious difference observed in the cathodic peaks (3.699 V). Furthermore,there is no significant intensity fading among the first,second and 40th cycle, demonstrating the good reversibility of the Li+ ions in the compound during the intercalating and de-intercalating processes.
|
Download:
|
| Fig. 4. Cyclic voltammograms of LiNi0.6Co0.2Mn0.2O2 cell at a scan rate of 0.1 mV/s. | |
In summary,the spherical Ni0.6Co0.2Mn0.2(OH)2 precursor with Ni and Mn concentration-gradient was successfully prepared by a hydroxide co-precipitated method. Well-ordered layered Li[Ni0.6Co0.2Mn0.2]O2 powders were successfully synthesized by mixing the as-prepared precursor and 5% excess LiOH·H2O. The sample delivered an initial discharge capacity of 174.3 mAh/g and a capacity retention of 93.1% after 100 cycles when cycled at 1 C between 2.8 V and 4.3 V,as well as excellent rate capability,high cut-off voltage and temperature performance. Therefore,the concentration-gradient structured LiNi0.6Co0.2Mn0.2O2 cathode material prepared by co-precipitation is a promising cathode material for lithium batteries,which is better than the similar samples containing the same amount of Ni reported before.
| [1] | W.M. Liu, G.R. Hu, Z.D. Peng, et al., Synthesis of spherical LiNi0.8Co0.15Al0.05O2 cathode materials for lithiuμ-ion batteries by a co-oxidation-controlled crystallization method, Chin. Chem. Lett. 22 (2011) 1099-1102. |
| [2] | C. Delmas, M. Ménétrier, L. Croguennec, et al., An overview of the Li(Ni,M)O2 systems: syntheses, structures and properties, Electrochim. Acta 45 (1999) 243-253. |
| [3] | I. Koetschau, M.N. Richard, J.R. Dahn, Orthorhombic, LiMnO2 as a high capacity cathode for Li-Ion Cells, J. Electrochem. Soc. 142 (1995) 2906-2910. |
| [4] | A.D. Robertson, S.H. Lu, W.F. Averill, J. Howard, M3+-Modified LiMn2O4 spinel intercalation cathodes I. Admetal effects on morphology and electrochemical performance, J. Electrochem. Soc. 144 (1997) 350'-3505. |
| [5] | T. Ohzuku, A. Ueda, M. Kouguchi, Synthesis and characterization of LiAl1/4Ni3/4O2(R3m) for lithiuμ-ion (Shuttlecock) batteries, J. Electrochem. Soc. 142 (1995) 4033-4039. |
| [6] | M. Yoshio, H. Noguchi, J. Itoh, M. Okada, T. Mouri, Preparation and properties of LiCoyMnxNi1xyO2 as a cathode for lithium ion batteries, J. Power Sources 90 (2000) 176-181. |
| [7] | W.B. Luo, F. Zhou, X.M. Zhao, Synthesis, characterization, and thermal stability of LiNi1/3Mn1/3Co1/3-zMgzO2, LiNi1/3-zMn-Co1/3-zMgzO2, and LiNi1/3Mn1/3-zCo1/3MgzO2, Chem. Mater. 22 (2010) 1164-1172. |
| [8] | Y.K. Sun, Z.H. Chen, H.J. Noh, et al., Nanostructured high-energy cathode materials for advanced lithium batteries, Nat. Mater. 11 (2012) 942-947. |
| [9] | Z.L. Huang, J. Gao, X.M. He, J.J. Li, C.Y. Jiang, Well-ordered spherical LiNixCo(1-2x)MnxO2 cathode materials synthesized from cobolt concentrationgradient precursors, J. Power Sources 202 (2012) 284-290. |
| [10] | G.M. Koenig Jr., I. Belharouak, H. Deng, Y.K. Sun, K. Amine, Composition-tailored synthesis of gradient transition metal precursor particles for lithiuμ-ion battery cathode materials, Chem. Mater. 23 (2011) 1954-1963. |
| [11] | H. Cao, Y. Zhang, J. Zhang, B.J. Xia, Synthesis and electrochemical characteristics of layered LiNi0.6Co0.2Mn0.2O2 cathode material for lithium ion batteries, Solid State Ionics 176 (2005) 1207-1211. |
| [12] | J.G. Li, L. Wang, Q. Zhang, X.M. He, Synthesis and characterization of LiNi0.6Mn0.4-xCoxO2 as cathode materials for Li-ion batteries, J. Power Sources 189 (2009) 28-33. |
| [13] | P.Y. Liao, J.G. Duh, S.R. Sheen, Microstructure and electrochemical performance of LiNi0.6Co0.4-xMnxO2 cathode materials, J. Power Sources 143 (2005) 212-218. |
| [14] | Y. Chen, G.X. Wang, K. Konstantinov, Synthesis and characterization of LiCoxMnyNi1-x-yO2 as a cathode material for secondary lithium batteries, J. Power Sources 119 (2003) 184-188. |
| [15] | Z.L. Liu, A.S. Yu, Y. Jim, Lee, Synthesis and characterization of LiNi1-x-yCoxMnyO2 as the cathode materials of secondary lithium batteries, J. Power Sources 81 (1999) 416-419. |
| [16] | P. Yue, Z.X. Wang, X.H. Li, The enhanced electrochemical performance of LiNi0.6Co0.2Mn0.2O2 cathode materials by low temperature fluorine substitution, Electrochim. Acta 95 (2013) 112-118. |
| [17] | S.K. Zhong, W. Li, Y.H. Li, X. Tang, Synthesis and electrochemical performances of LiNi0.6Co0.2Mn0.2O2 cathode materials, Trans. Nonferrous Met. Soc. China 19 (2009) 1499-1503. |
| [18] | Y. Zhang, H. Cao, J. Zhang, B.J. Xia, Synthesis of LiNi0.6Co0.2Mn0.2O2 cathode material by a carbonate co-precipitation method and its electrochemical characterization, Solid State Ionics 177 (2006) 3303-3307. |
| [19] | M. Dahbi, J.M. Wikberg, I. Saadoune, et al., Electrochemical behavior of LiNi1-y-zCoMnzO2 probed through structural and magnetic properties, J. Appl. Phys. 111 (2012) 023904. |
| [20] | P. Yue, Z.X. Wang, H.J. Guo, et al., Effect of synthesis routes on the electrochemical performance of Li[Ni0.6Co0.2Mn0.2]O2 for lithium ion batteries, J. Solid State Electrochem. 16 (2012) 3849-3854. |
| [21] | C.L. Gan, X.H. Hu, H. Zhan, Y.H. Zhou, Synthesis and characterization of Li1.2Ni0.6Co0.2Mn0.2O2+δ as a cathode material for secondary lithium batteries, Solid State Ionics 176 (2005) 687-692. |
| [22] | P. Yue, Z.X. Wang, W.J. Peng, et al., Spray-drying synthesized LiNi0.6Co0.2Mn0.2O2 and its electrochemical performance as cathode materials for lithium ion batteries, Powder Technol. 214 (2011) 279-282. |
| [23] | P. Yue, Z.X. Wang, W.J. Peng, et al., Preparation and electrochemical properties of submicron LiNi0.6Co0.2Mn0.2O2 as cathode material for lithium ion batteries, Scr. Mater. 65 (2011) 1077-1080. |




