Carbonaceous materials attracted much attention due to their diversities of their practical applications [1, 2, 3, 4, 5, 6]. During the last decades,carbonaceous materials with spherical,one- and twodimensional morphologies have been widely studied. The carbonaceous spheres can be prepared using block copolymers as the templates [4]. The carbonaceous nanofibers have been prepared using mesoporous silicas as the hard templates [7]. The carbonaceous nanotubes can be prepared by the carbonization of polymer nanotubes or using a CVD method [8, 9]. Among them, carbonaceous fibers and tubes with chiral structures developed very fast,which are potentially applied for electromagnetic wave absorption,asymmetric catalysis,supaercapacitor electrode and enantioseparation [9, 10, 11, 12, 13, 14, 15]. However,to obtain single-handed, helical,carbonaceous fibers,or tubes,is still very difficult.
Recently,it was reported that single-handed helical,N-doped, carbonaceous nanotubes could be prepared by the carbonization of polypyrrole [9]. The resulting samples exhibited interesting optical activity. Diffuse reflectance circular dichroism (DRCD) signals were centered at 400-500 nm. With the development of the sol-gel transcription approach,varieties of single-handed helical polysilsesquioxane nanofibers and nanotubes have been successfully prepared using chiral,low molecular weight gelators through the external,or self-templating approaches [16, 17, 18]. The circular dichroism (CD) characterization indicated that these materials exhibit chirality at Å ngstrom level. In addition,these chiral polysilsesquioxanes can be used as asymmetric catalysts [19]. Our recent results indicated that single-handed,helical,carbonaceous nanotubes could be also prepared from single-handed,helical, polybissilsesquioxane nanotubes by carbonization and the removal of silica [15]. The carbonaceous nanotubes also exhibited optical activity. DRCD signals were identified and centered at 380- 400 nm. However,the origin of the optical activity is still unclear. Herein,single-handed,coiled,carbonaceous,tubular nanoribbons were prepared through this approach,with helical pitches about 800-1600 nm. Positive DRCD signal centered at about 700 nm was identified. The helical pitch was proposed to play an important role in the position of the DRCD signal.
2. Experimental 2.1. General methodsTransmission electron microscopy (TEM) images were obtained using an FEI TecnaiG220 at 200 kV. Field-emission scanning electron microscopy (FE-SEM) was performed using a Hitachi 4800 instrument at 3.0 kV. Small angle X-ray diffraction (SAXRD) and wide angle X-ray diffraction (WAXRD) patternswere obtained using an X0 Pert-Pro MPD X-ray diffractometer using Cu Kα radiation with a Ni filter (1.542 Å ). Specific surface area and pore-size distribution were determined by the Brunauer-Emmett-Teller (BET) and Barrett-Joyner-Halon (BJH) methods using N2 adsorption isotherm measured by a Micromeritics Tristar II 3020 instrument. DRCD spectra were obtained using a JASCO 815 spectrophotometer. Raman spectra were obtained using a Jobin Yvon Horiba HR 800 Labramconfocal microprobe Raman system. The sizes of the silt and pinhole were 100 μm and 400 μm,respectively. The Ar laser excitation wavelength was 633 nm,and largest laser power was 10 mW.
2.2. MaterialsThe preparation procedure for the left-handed,coiled,4,4'- biphenylene bridged polybissilsesquioxane,tubular nanoribbons has been reported previously [20].
Synthetic procedure for the left-handed,coiled,carbon/silica, tubular nanoribbons: The left-handed,coiled,4,4'-biphenylene bridged polybissilsesquioxane,tubular nanoribbons were carbonized at 900 ℃ for 4.0 h with a heating rate of 3.0 ℃/min in Ar. The carbon/silica,tubular nanoribbons were obtained by cooling to room temperature naturally.
Synthetic procedure for the left-handed,coiled,carbonaceous, tubular nanoribbons: The above resulting left-handed,coiled, carbon/silica,tubular nanoribbons were immersed in 10 wt% aqueous HF for 4.0 h and then washed with deionized water. Sample was dried in vacuum at 40 ℃ overnight.
3. Results and discussionThe left-handed,coiled,4,4'-biphenylene bridged polybissilsesquioxane, tubular nanoribbons were prepared by the selfassembly of an anionic gelator as the template,3-aminopropyltrimethoxysilane as a co-structure directing agent and 4,4'- bis(triethoxysilyl)-1,1'-biphenyl as the precursor has been reported previously [20]. After carbonization,left-handed,coiled, carbon/silica nanotubes were obtained,which are 40-400 nm wide and 30 nm thick (Fig. 1a). The TEM image indicated that the nanotubes were constructed as double layers of nanoribbons (Fig. 1c). The interlayer distance is about 4.0 nm. After removal of silica using a HF aqueous solution,left-handed,coiled,carbonaceous, tubular nanoribbons were obtained (Figs. 1b,d,and e). The width and thickness did not change significantly. The helical pitches were about 800-1600 nm. However,few of the double layers of nanoribbons merged together (Fig. 1d). Micropores were identified in Fig. 1d,which were formed due to the removal of silica [21].

|
Download:
|
| Fig. 1. FESEM (a) and TEM (c) images of the left-handed,coiled,carbon/silica, tubular nanoribbons; FESEM (b) and TEM (d and e) images of the left-handed, coiled,carbonaceous,tubular nanoribbons. | |
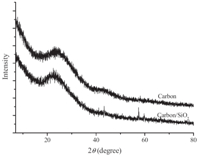
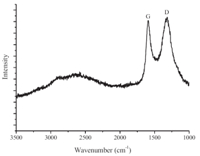
WAXRD patterns of left-handed,coiled,tubular nanoribbons are shown in Fig. 2. Before calcination,the pattern of left-handed, coiled,4,4'-biphenylene bridged polybissilsesquioxane,tubular nanoribbons show peaks at 2θ of 7.18,15.28,and 23.08,indicating a lamellar structure with a 12.4Å periodicity [20, 22]. After carbonization,two broad peaks were identified at 2θ of 22.78 and 43.38,indicating no large crystalline domains within the sample (Fig. 2). The lamellar periodicity was damaged. When the left-handed,coiled,4,4'-biphenylene bridged polybissilsesquioxane, tubular nanoribbons were calcined at 900 ℃ for 4.0 h in Ar,the biphenylene moieties were converted into carbon by this process due to pyrolysis of many biphenyl species [22]. Compared with the original structure,the structure was distorted and the periodicity was destroyed. The broad peak at 22.7° originated from the carbon and silica frame work,and the peak at 43.3° originated from the carbon frame work,indicating that atoms reorganized during carbonization. After removing silica,two broad peaks were identified at 2θ of 24.2° and 43.38. This suggests the walls were predominantly amorphous carbon,or the crystalline size was very small [14]. Raman spectra of left-handed,helical,carbonaceous, tubular nanoribbons at 633 nm excitation are shown in Fig. 3. The D and G bands were observed at 1323 and 1594 cm-1,respectively. The IG/ID ratios indicated the crystalline sizes were less than several nanometers [14].
|
Download:
|
| Fig. 2. WAXRD pattern of the carbon/silica and carbonaceous,tubular nanoribbons. | |
|
Download:
|
| Fig. 3. Raman spectra of the left-handed helical,carbonaceous,tubular nanoribbons. | |
For 4,4'-biphenylene-silica,tubular nanoribbons,it was reported that the CD spectrum shows two negative signals at 226 and 295 nm and one positive signal at 258.5 nm [20]. The signal at 295 nm is originated from the right-handed twist of the biphenylene rings (Fig. 4a). DRCD and DRUV-vis spectra of the single-handed,helical,4,4'-biphenylene-silica,carbon/silica and carbonaceous nanotubes and single-handed helical,carbonaceous, tubular nanoribbons are shown in Fig. 4b. For 4,4'-biphenylenesilica, tubular nanoribbons,a UV absorbance band was identified at 29° nmand a negative DRCD signal was identified at 304 nm,those were originated from the right-handed,twisted,biphenylene rings. For the carbon/silica and carbonaceous,tubular nanoribbons, broad UV-vis absorbance bands were identified from 250 to 800 nm. Moreover,broad,positive DRCD signals were identified from 350 nm to 800 nm. Single-handed,helical,carbonaceous nanotubes with DRCD signals centered at about 380-400 nm have been prepared according to a similar synthetic approach [15]. The helical pitches are about 600-800 nm. Herein,the DRCD signal of the carbonaceous,tubular nanoribbons shifted to longer wavelength. The helical pitches are about 800-1600 nm (Fig. 1b). The helical pitch seems to play an important role in the position of the DRCD signals [23].

|
Download:
|
| Fig. 4. (a) CD spectra of the 4,4'-biphenylene-silica,tubular nanoribbons and (b) DRCD spectra of the single-handed,helical,4,4'-biphenylene bridged polybissilsesquioxane, carbon/silica and carbonaceous,tubular nanoribbons. | |
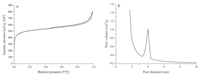
The nitrogen sorption measurements are shown in Fig. 5. Fig. 5a shows nitrogen adsorption-desorption isothermal plots for the left-handed,coiled,carbonaceous,tubular nanoribbons. The three samples showed one broad hysteresis loop at a relative pressure (P / P0) between 0.45 and 1.0,which originated from voids within and among the nanotubes. Micropores were also identified from the isotherm plots,which should be formed due to the removal of silica [21]. The BJH pore size distribution plot determined from the desorption branch is shown in Fig. 5b. The distance between the double layers of carbonaceous ribbons should be about 4.0 nm, which is consistence with the TEM image (Fig. 1e). The nitrogen BET surface area is 1727 m2/g.
|
Download:
|
| Fig. 5. (a) Sorption isotherms and (b) BJH pore size distribution plots calculated from the adsorption branch. D. Liu et al. / Chinese Chemical Letters 25 (2014) 879-882 881 | |
Left-handed,coiled,carbonaceous,tubular nanoribbons were prepared by carbonization and removal of silica using left-handed, coiled,4,4'-biphenylene bridged polybissilsesquioxane,tubular nanoribbons as the raw materials. A broad,positive DRCD signal was identified centered at 700 nm,indicating the carbonaceous tubular nanoribbons exhibit optical activity. Compared with previous results,the helical pitch is proposed to play an important role in the position of the DRCD signal. The nitrogen BET surface area is 1727m2/g. This kind of carbonaceous materials is potentially applied as catalyst support and supercapacitor electrodes. Moreover, single-handed helical,mesoporous,carbon/silica and carbonaceous nanofibers were also potentially prepared through the approach shown here,which might exhibit optical chirality.
AcknowledgmentsThis work was supported by Natural Science Foundation of Jiangsu Province (No. BK2011354),the Priority Academic Program Development of Jiangsu High Education Institutions (PAPD),and the National Natural Science Foundation of China (Nos. 21104053, 21071103 and 21074086).
| [1] | R. Ryoo, S.H. Joo, S. Jun, Synthesis of highly ordered carbon molecular sieves via template-mediated structural transformation, J. Phys. Chem. B 103 (1999) 7743-7746. |
| [2] | Y. Meng, D. Gu, F.Q. Zhang, et al., Ordered mesoporous polymers and homologous carbon frameworks: amphiphilic surfactant templating and direct transformation, Angew. Chem. Int. Ed. 44 (2005) 7053-7059. |
| [3] | Y. Fang, Y.Y. Lü, R.C. Che, et al., Two-dimensional mesoporous carbon nanosheets and their derived graphene nanosheets: synthesis and efficient lithium ion storage, J. Am. Chem. Soc. 135 (2013) 1524-1530. |
| [4] | Y. Fang, D. Gu, Y. Zou, et al., A low-concentration hydrothermal synthesis of biocompatible ordered mesoporous carbon nanospheres with tunable and uniform size, Angew. Chem. Int. Ed. 49 (2010) 7987-7991. |
| [5] | Y.Y. Lü, F. Zhang, Y.Q. Dou, et al., A comprehensive study on KOH activation of ordered mesoporous carbons and their supercapacitor application, J. Mater. Chem. 22 (2012) 93-99. |
| [6] | Y.P. Zhai, Y.Q. Dou, D.Y. Zhao, et al., Carbon materials for chemical capacitive energy storage, Adv. Mater. 23 (2011) 4828-4850. |
| [7] | C.Z. Yu, J. Fan, B.Z. Tian, D.Y. Zhao, G.D. Stucky, High-yield synthesis of periodic mesoporous silica rods and their replication to mesoporous carbon rods, Adv. Mater. 14 (2002) 1742-1745. |
| [8] | W. Yang, W.J. Sun, W. Chu, C.F. Jiang, J. Wen, Synthesis of carbon nanotubes using scrap tyre rubber as carbon source, Chin. Chem. Lett. 23 (2012) 363-366. |
| [9] | S.H. Liu, Y.Y. Duan, X.J. Feng, J. Yang, S.A. Che, Synthesis of enantiopure carbonaceous nanotubes with optical activity, Angew. Chem. Int. Ed. 52 (2013) 6858-6862. |
| [10] | S. Motojima, S. Hoshiya, Y. Hishikawa, Electromagnetic wave absorption properties of carbon microcoils/PMMA composite beads in W bands, Carbon 41 (2003) 2658-2660. |
| [11] | Y. Qin, Z.K. Zhang, Z.L. Cui, Helical carbon nanofibers prepared by pyrolysis of acetylene with a catalyst derived from the decomposition of copper tartrate, Carbon 41 (2003) 3072-3074. |
| [12] | Y. Qin, Z.K. Zhang, Z.L. Cui, Helical carbon nanofibers with a symmetric growth mode, Carbon 42 (2004) 1917-1922. |
| [13] | Y. Qin, L.Y. Yu, Y. Wang, G.C. Li, Z.L. Cui, Amorphous helical carbon nanofibers synthesized at low temperature and their elasticity and processablity, Solid. State. Commun. 138 (2006) 5-8. |
| [14] | X.Q. Chen, S.M. Yang, S.J. Motojima, M. Ichihara, Morphology and microstructure of twisting nano-ribbons prepared using sputter-coated Fe-base alloy catalysts on glass substrates, Mater. Lett. 59 (2005) 854-858. |
| [15] | C.Y. Zhang, Y. Li, B.Z. Li, Y.G. Yang, Preparation of single-handed helical carbon/silica and carbonaceous nanotubes by using 4,40-biphenylene-bridged polybissilsesquioxane, Chem. Asian J. 8 (2013) 2714-2720. |
| [16] | J.J.E. Moreau, L. Velutini, M.W.C. Man, C. Bied, New hybrid organic-inorganic solids with helical morphology via H-bond mediated sol-gel hydrolysis of silyl derivatives of chiral (R,R)-or (S,S)-diureidocyclohexane, J. Am. Chem. Soc. 123 (2001) 1509-1510. |
| [17] | X.J. Wu, S.J. Ji, Y. Li, et al., Helical transfer through nonlocal interactions, J. Am. Chem. Soc. 131 (2009) 5986-5993. |
| [18] | B.Z. Li, Z. Xu, W. Zhuang, et al., Characterization of 4,40-biphenylene-silicas and a chiral sensor for silicas, Chem. Commun. 47 (2011) 11495-11497. |
| [19] | A. Brethon, J.J.E. Moreau, M.W.C. Man, Chiral hybrid silica sol-gel heterogenisation of trans-(1R, 2S)-diaminocyclohexane ligands for the rhodium catalysed enantioselective reduction of acetophenone, Tetrahedron: Asymmetry 15 (2004) 495-502. |
| [20] | H.T. Li, B.Z. Li, Y.L. Chen, et al., Preparetion of chiral 4,40-biphenylene-silica nanoribbons, Chin. J. Chem. 27 (2009) 1860-1862. |
| [21] | Z.W. Wu, J.B. Pang, Y.F. Lu, Synthesis of highly-ordered mesoporous carbon/silica nanocomposites and derivative hierarchically mesoporous carbon from a phenylbridged organosiloxane, Nanoscale 1 (2009) 245-249. |
| [22] | M.P. Kapoor, Q.H. Yang, S.J. Inagaki, Self-assembly of biphenylene-bridged hybrid mesoporous solid with molecular-scale periodicity in the pore walls, J. Am. Chem. Soc. 124 (2002) 15176-15177. |
| [23] | J.J. Xie, H.B. Qiu, S.A. Che, Handedness inversion of chiral amphiphilic molecular assemblies evidenced by supramolecular chiral imprinting in mesoporous silica assemblies, Chem. Eur. J. 18 (2012) 2559-2564. |




