Over the past decades,considerable attentions have been paid to rational design and synthesis of novel coordination polymers with intriguing structure and diverse functions [1, 2],owing to its promising application as functional materials in many fields [3, 4]. As two typical bridging groups affording magnetic clusters and polymers,carboxylate and azido anions have been widely used due to their rich coordination modes as well as diverse magnetic coupling patterns [5, 6, 7],and therefore many unique carboxylatecontaining ligands have been developed to construct magnetic coordination compounds.
Recently,some coordination compounds derived from azide and zwitterionic dicarboxylate ligands were reported [7],which demonstrates that using zwitterionic carboxylates as ligands is an efficient synthetic strategy toward mixed azide- and carboxylatebridging systems [7, 8]. Normally when zwitterionic carboxylates are used as neutral organic ligands to construct metal-organic compounds,additional anions are necessary for charge balance [9]. Compared with rigid ligands,flexible ligands can form some unique and interesting frameworks because it can freely bend and rotate in the assembly process [8a, 10].
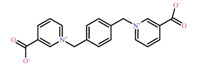
In this paper,we selected 1,4-bis(pyridinil-3-carboxylato)-l,4-dimethylbenzene (L) as organic linker (Scheme 1) and azide as co-ligand to produce a novel 2-fold interpenetrating three dimension (3D) Mn(II) polymer {[Mn2L(N3)4].2H2O}n(1)with anti-ferromagnetism.
|
Download:
|
| Scheme 1.The structure of L [L =1,4-bis(pyridinil-3-carboxylato)-1,4-dimethylbenzene] | |
Materials and physical measurements: 1,4-Bis(pyridinil-3-carboxylato)-l,4-dimethylbenzene (L) was prepared according to the literature [11]. Other chemicals are commercially available and were used as received. Elemental analysis was carried on a PerkinElmer 240C analyzer. IR spectrum was measured on a Tensor 27 (Bruker) FT-IR spectrometer with KBr pellets. The X-ray powder diffraction (XRPD) experiments were carried out on a Rigaku D/ Max-2500 diffractometer,operated at 40 kV and 100 mA,using a Cu-target tube and a graphite monochromator. Simulation of the PXRD spectra were conducted based on the single-crystal data and diffraction-crystal module of the Mercury (Hg) program available free of charge in Internet (http://www.iucr.org). Magnetic data were collected using crushed crystals of the sample on a Quantum Design MPMS-XL SQUID magnetometer.
Synthesis of L:L was prepared in a way similar to that reported 20 mmol). The mixture was refluxed and filtered to give a white precipitate,which was further hydrolyzed by dilute hydrochloric acid (5%,50 mL). Then,the bromide ions were removed by fresh silver(I) oxide (prepared by the reaction of AgNO3 and NaOH in aqueous solution),and white powder of L was obtained (Yield: 80% based on 1,4-bis-bromomethylbenzene). Anal. calcd. for C20H16N2O4(%): C 68.96,H 4.63,N 8.04. Found (%): C 68.82,H 4.68,N 8.13. IR (KBr,cm-1): 3342(w),3288(w),3037(m),1728(m), 1640(m),1301(s),1130(s),750(s),671(s).
Synthesis of {[Mn2L(N3)4].2H2O}n (1): Mn(ClO4)2.6H2O (0.2 mmol),L (0.15 mmol) and NaN3(1 mmol) were added to a mixture solution of C2H5OH (4 mL) and H2O (2 mL). The resulting mixture was sealed in a Teflon-lined autoclave,and heated at 75°C for 24 h. After cooling to room temperature,yellow block crystal was obtained with a yield of 80% based on L. FT-IR (KBr pellet, cm-1): 3607(s),3384(m),3302(m),2080(s),1635(m),1602(w), 1406(w),1384(s). Element. Anal. Calcd. for C20H20Mn2N14O6(%): C 36.25,H 3.02,N 29.61; Found (%): C 36.22,H 3.01,N 29.59.
Caution! Azido compounds of metal ions are potentially explosive, and only a small amount of materials should be prepared with care.
Cystallographic Studies: X-ray diffraction data were collected
on a SCX-mini diffractometer at 293 K with graphite monochromated Mo-Karadiation ( = 0.71073
= 0.71073  )byanvscan mode. The
program SAINT [12] was used for the integration of the diffraction
profiles. Absorption corrections were carried out by using multiscan program SADABS [13]. The structures were solved by direct
method and refined by full-matrix least-squares technique using
SHELXTL [14]. The positions of metal atoms were located fromEmaps by direct-method and other non-hydrogen atoms were
refined with anisotropic displacement parameters. The hydrogen
atoms of the ligands were generated theoretically onto specific
atoms and refined isotropically with fixed thermal factors.
Crystallographic data and structure refinement results are
summarized in Table 1 and selected bond lengths and angles
are listed in Table S1 in Supporting information. Crystallographic
data (excluding structure factors) for 1 have been deposited in the
Cambridge Crystallographic Data Centre,CCDC,12 Union Road,
Cambridge CB21EZ,UK. Copies of the data can be obtained free of
charge on quoting the depository number CCDC-996878,the
names of the authors,(E-mail: deposit@ccdc.cam.ac.uk,http://www.ccdc.cam.ac.uk).
)byanvscan mode. The
program SAINT [12] was used for the integration of the diffraction
profiles. Absorption corrections were carried out by using multiscan program SADABS [13]. The structures were solved by direct
method and refined by full-matrix least-squares technique using
SHELXTL [14]. The positions of metal atoms were located fromEmaps by direct-method and other non-hydrogen atoms were
refined with anisotropic displacement parameters. The hydrogen
atoms of the ligands were generated theoretically onto specific
atoms and refined isotropically with fixed thermal factors.
Crystallographic data and structure refinement results are
summarized in Table 1 and selected bond lengths and angles
are listed in Table S1 in Supporting information. Crystallographic
data (excluding structure factors) for 1 have been deposited in the
Cambridge Crystallographic Data Centre,CCDC,12 Union Road,
Cambridge CB21EZ,UK. Copies of the data can be obtained free of
charge on quoting the depository number CCDC-996878,the
names of the authors,(E-mail: deposit@ccdc.cam.ac.uk,http://www.ccdc.cam.ac.uk).
| Table 1 Crystal data and structure refinement parameters for polymer 1. |
Single-crystal X-ray diffraction analysis reveals that1crystallized in triclinicP-1 space group. The asymmetric unit comprises of
one crystallographically independent Mn(II) ion (Mn1),two
independent halves of Mn(II) ions (Mn2 and Mn3),four azido
anions,one L ligand and two uncoordinated water molecules,as
displayed in Fig. 1a. Mn1 locates in a disordered coordination
environment defined by four azido nitrogen atoms (N1,N4,N7 and
N10) and twociscarboxylate oxygen atoms (O2 and O3). Mn2 and
Mn3 both assume thetrans-octahedral [N4O2] coordination
environment defined by four equatorial azido nitrogen atoms
(N7,N7B,N10 and N10B for Mn2,N1,N1A,N4 and N4A for Mn3)
and two axial carboxylate oxygen atoms (O4 and O4B for Mn2,O1
and O1A for Mn3) (Fig. 1b). The Mn-N distances for Mn2 and Mn3
(2.195(4)-2.271(4)  for Mn2 and 2.213(3)-2.214(3)
for Mn2 and 2.213(3)-2.214(3) 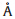 for Mn3)
are slightly longer than the Mn-O distances (2.167(3)
for Mn3)
are slightly longer than the Mn-O distances (2.167(3) 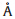 for Mn2
and 2.211(3)
for Mn2
and 2.211(3) 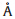 for Mn3),indicating an axial elongation of the sequence and two adjacent metal ions are triply bridged by two EO
(EO = end-on) azido ions and one syn-syn carboxylate group,to
generate a uniform chain (Fig. 1b).
for Mn3),indicating an axial elongation of the sequence and two adjacent metal ions are triply bridged by two EO
(EO = end-on) azido ions and one syn-syn carboxylate group,to
generate a uniform chain (Fig. 1b).

|
Download:
|
| Fig. 1. View of (a) the asymmetric unit of polymer1and (b) the coordination environment of Mn(II) and 1D chain. Hydrogen atoms and water molecules are omitted for clarity. Symmetry codes: (A)-x+2,-y, -z+ 1. (B)-x+2,-y, -z. (C) -x+1,-y, -z + 2. (D)-x+1,-y+1,-z+1. | |
For the L in 1,the two pyridinium rings adopts trans conformation with respect to the 1,4-dimenthylbenzene,leading to a zigzag shape for the ligand. Each chain is linked with adjacent four identical chains through L ligands to give a complicated 3D network (Fig. 2a). Large ‘‘empty’’ space related to the L linkers is observed for the single network,but is filled by the 2-fold interpenetration occurred between two equivalent 3D networks (Fig 2b).

|
Download:
|
| Fig. 2. (a) Single 3D network. (b) The two-fold interpenetration of network. | |
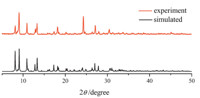
The X-ray powder diffraction (XRPD) experiment was performed in order to make sure that the crystal structure is truly representative of the bulk materials. The diffraction intensity data were recorded by continuous scan in a 2θ/θ mode from 5° to 50° with a step size of 0.02° and a scan speed of 8° min-1 . The experimental diffraction pattern and the simulated pattern are shown in Fig. 3. Good consistence between the experimental pattern and the simulated pattern as displayed in Fig. 3 indicates that bulk-synthesized materials and the as-grown crystals are homogeneous.
|
Download:
|
| Fig. 3. X-ray powder diffraction (XRPD) patterns for 1. | |
The solid state magnetic susceptibility of polymer 1 was measured in 2-300 K at a field of 1 kOe,and the dependence of magnetic susceptibility on temperature was plotted inxm T vs T profile (Fig. 4a) and xm-1 vs T(Fig. S1 in Supporting information). The xm T value per Mn(II) at 300 K (3.96 emu K mol-1) is lower than the spin-only value (4.38 emu K mol-1) expected for a magnetically isolated high-spin Mn(II) ion (g= 2.0),which reveals the presence of anti-ferromagnetic (AF) coupling in this polymer. The shape of xm T plot (Fig. 4a) implies the AF interaction between the Mn(II) ions in 1,which is corroborated by the large negative Weiss constant θ=-68 K deduced from the Curie-Weiss fitting of the xm-1vs T data above 50 K (Fig. S1). The field-dependent magnetization at 2 K shows a linear increasing trend and reaches 0.61 Nbat 7 T (Fig. 4b),which is much smaller than the saturated vale 5 Nbexpected for one Mn(II) ion with g= 2.0 and S= 5/2 and further confirms the AF coupling of 1.

|
Download:
|
| Fig. 4. (a) The xm T vs T plot of 1. The solid red lines represent the best fits to the uniform-chain model. (b) TheMvsHplot of 1. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) | |
The global AF behavior of 1 can be interpreted by magnetostructural relationship. The Mn(II) ions in1are bridged by EO azido andsyn,syncarboxylate of L ligand. It is well known thatsyn,syn carboxylate mediates AF interaction,while the nature of the coupling transmitted by EO mode depends on the M-Nazido-M angle [5c]. For Mn(II) ions,the ferromagnetic coupling appears over the crossover angle of 98° and reaches its top at 106° [5c]. In polymer1,the bridging angle of EO azido is 94.69° for Mn(1)-N(4)- Mn(3) and 95.08° for Mn(1)-N(1)-Mn(3); while the bridging angle of EO azido is 94.27° for Mn(1)-N(7)-Mn(2) and 93.12° for Mn(1)- N(10)-Mn(2). All of these angles are smaller than 98° and therefore conduct AF coupling. The AF exchange interaction mediated by EO azido andsyn,syncarboxylate of L ligand should account for the global AF behavior of 1.
Complex 1 can be magnetically considered as an infinite
uniform chain in which the magnetic coupling is mediated through
the mixed (μ-EO-N3)2(μ-COO) triple bridges. The interchain
interactions viathe long L ligand could be ignored. Considering
that the chain contains two sets of triple bridges with different
structural parameters that alternating in AABB sequence,it
should be described as a 1D Heisenberg chain with alternating
J1-J1-J2-J2 interactions. The corresponding Hamiltonian is
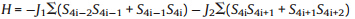 and the
expression ofxfor such a chain in the classical-spin approximation
was exported as follows [15]:
and the
expression ofxfor such a chain in the classical-spin approximation
was exported as follows [15]:
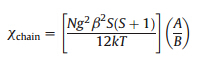
A Mn(II) coordination polymer with a 2-fold interpenetrating 3D framework based on azide ion and zwitterionic dicarboxylate was synthesized under hydrothermal conditions. Furthermore, magnetism analysis reveals anti-ferromagnetism for 1. Acknowledgment
This work was financially supported by MOE Innovation Team of China (No. IRT13022). Appendix A. Supplementary data
Supplementary material related to this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.05.026.
| [1] | (a) B. Moulton, M.J. Zaworotko, From molecules to crystal engineering: supramolecular isomerism and polymorphism in network solids, Chem. Rev. 101 (2001) 1629-1658;(b) D. Tian, Q. Chen, Y. Li, et al., A mixed molecular building block strategy for the design of nested polyhedron metal-organic frameworks, Angew. Chem. Int. Ed. 53 (2014) 837-841;(c) J.R. Li, Y. Tao, Q. Yu, et al., Selective gas adsorption and unique structural topology of a highly stable guest-free zeolite-type MOF material with N-rich chiral open channels, Chem. Eur. J. 14 (2008) 2771-2776;(d) S.R. Batten, K.S. Murray, Structure and magnetism of coordination polymers containing dicyanamide and tricyanomethanide, Coord. Chem. Rev. 246 (2003) 103-130;(e) K.H. He, Y.W. Li, Y.Q. Chen, Z. Chang, A new 8-connected self-penetrating metal-organic framework based on dinuclear cadmiumclusters as secondary building units, Chin. Chem. Lett. 24 (2013) 691-694. |
| [2] | (a) K. Sumida, D.L. Rogow, J.A. Mason, et al., Carbon dioxide capture in metal-organic frameworks, Chem. Rev. 112 (2012) 724-789;(b) T.R. Cook, Y.R. Zheng, P.J. Stang, Metal-organic frameworks and self-assembled supramolecular coordination complexes: comparing and contrasting the design, synthesis, and functionality of metal-organic materials, Chem. Rev. 113 (2013) 734-777;(c) G. Férey, C.Mellot-Draznieks, C. Serre, F. Millange, Crystallized frameworkswith giant pores: are there limits to the possible? Acc. Chem. Res. 38 (2005) 217-225;(d) G.Q. Kong, C.D. Wu, A self-assembled supramolecular solid for catalytic application, Inorg. Chem. Commun. 12 (2009) 731-734. |
| [3] | (a) S.L. James, Metal-organic frameworks, Chem. Soc. Rev. 32 (2003) 276-288;(b) H.L. Jiang, T. Akita, T. Ishida, M. Haruta, Q. Xu, Synergistic catalysis of Au@Ag core-shell nanoparticles stabilized on metal-organic framework, J. Am. Chem. Soc. 133 (2011) 1304-1306;(c) Y. Mizuno, M. Okubo, K. Kagesawa, et al., Precise electrochemical control of ferromagnetism in a cyanide-bridged bimetallic coordination polymer, Inorg. Chem. 51 (2012) 10311-10316;(d) F. Pointillart, K. Bernot, G. Poneti, R. Sessoli, Crystal packing effects on the magnetic slow relaxation of Tb(III)-nitronyl nitroxide radical cyclic dinuclear clusters, Inorg. Chem. 51 (2012) 12218-12229;(e) G.Q. Kong, C.D. Wu, Four novel coordination polymers based on a flexible zwitterionic ligand and their framework dependent luminescent properties, Cryst. Growth Des. 10 (2010) 4590-4595. |
| [4] | (a) J.Y. Lee, O.M. Farha, J. Roberts, et al., Metal-organic framework materials as catalysts, Chem. Soc. Rev. 38 (2009) 1450-1459;(b) A. Corma, H. Garcia, F.X. Llabres, I. Xamena, Engineering metal organic frameworks for heterogeneous catalysis, Chem. Rev. 110 (2010) 4606-4655;(c) F.J. Song, C. Wang, J.M. Falkowski, L.Q. Ma, W.B. Lin, Isoreticular chiral metal-organic frameworks for asymmetric alkene epoxidation: tuning catalytic activity by controlling framework catenation and varying open channel sizes, J. Am. Chem. Soc. 132 (2010) 15390-15398;(d) K.K. Tanabe, S.M. Cohen, Engineering a metal-organic framework catalyst by using postsynthetic modification, Angew. Chem. Int. Ed. 48 (2009) 7424-7427;(e) C.M. Liu, D.Q. Zhang, D.B. Zhu, Field-induced single-ion magnets based on enantiopure chiral β-diketonate ligands, Inorg. Chem. 52 (2013) 8933-8940. |
| [5] | (a) J. Ribas, A. Escuer, M. Monfort, et al., Polynuclear Ni(II) and Mn(II) azido bridging complexes. Structural trends and magnetic behavior, Coord. Chem. Rev. 193 (1999) 1027-1068;(b) X.Y. Wang, Z.M. Wang, S. Gao, Constructing magnetic molecular solids by employing three-atom ligands as bridges, Chem. Commun. (2008) 281-294;(c) Y.F. Zeng, X. Hu, F.C. Liu, X.H. Bu, Azido-mediated systems showing different magnetic behaviors, Chem. Soc. Rev. 38 (2009) 469-480;(d) F.C. Liu, Y.F. Zeng, J.R. Li, et al., Novel 3-D framework nickel(II) complex with azide, nicotinic acid, and nicotinate(1) as coligands: hydrothermal synthesis, structure, and magnetic properties, Inorg. Chem. 44 (2005) 7298-7300;(e) H.H. Ko, J.H. Lim, H.C. Kim, C.S. Hong, Coexistence of spin canting and metamagnetism in a one-dimensional Mn(III) complex bridged by a single end-to-end azide, Inorg. Chem. 45 (2006) 8847-8849. |
| [6] | (a) E. Ruiz, J. Cano, S. Alvarez, P. Alemany, Magnetic coupling in end-on azidobridged transition metal complexes: a density functional study, J. Am. Chem. Soc. 120 (1998) 11122-11129;(b) E.C.Yang, Z.Y. Liu,Z.Y. Liu, L.N.Zhao,X.J. Zhao, Long-range ferromagneticordering in a 3D Cu(II)-tetracarboxylate framework assisted by an unprecedented bidentate μ2-O1,N4 hypoxanthine nucleobase, Dalton Trans. 39 (2010) 8868-8871;(c) C.S. Hong, J.E. Koo, S.K. Son, et al., Unusual ferromagnetic couplings in single endto-end azide-bridged cobalt (II) and nickel (II) chain systems, Chem. Eur. J. 7 (2001) 4243-4252;(d) T.C. Stamatatos, G. Christou, Azide groups in higher oxidation state manganese cluster chemistry: from structural aesthetics to single-molecule magnets, Inorg. Chem. 48 (2009) 3308-3322;(e) H.R.Wen, C.F.Wang, Y. Song, J.L. Zuo, X.Z. You, One-dimensional azido-bridged chiral metal complexes with ferromagnetic or antiferromagnetic interactions: syntheses, structures, and magnetic studies, Inorg. Chem. 44 (2005) 9039-9045. |
| [7] | (a) X.B. Li, Y. Ma, X.M. Zhang, J.Y. Zhang, E.Q. Gao, Azide-bridged copper (II) and manganese(II) compounds with a zwitterionic tetrazolate ligand: structures and magnetic properties, Eur. J. Inorg. Chem. 30 (2011) 4738-4744;(b) F.C. Liu, Y.F. Zeng, J. Jiao, et al., First metal azide complex with isonicotinate as a bridging ligand showing new net topology: hydrothermal synthesis, structure, and magnetic properties, Inorg. Chem. 45 (2006) 2276-2778. |
| [8] | (a) Y.F. Zeng, F.C. Liu, J.P. Zhao, et al., An azido-metal-isonicotinate complex showing long-range ordered ferromagnetic interaction: synthesis, structure and magnetic properties, Chem. Commun. (2006) 2227-2229;[(Fig._4)TD$FIG] Fig. 4. (a) The xmT vs T plot of 1. The solid red lines represent the best fits to the uniform-chain model. (b) TheMvsHplot of 1. (For interpretation of the references to color in this figure legend, the reader is referred to the web version of this article.) R.-M. Wen et al. /Chinese Chemical Letters 25 (2014) 854-858 857 (b) Y.F. Zeng, J.P. Zhao, B.W. Hu, et al., Structures with tunable strong ferromagnetic coupling: from unordered (1D) to ordered (discrete), Chem. Eur. J. 13 (2007) 9924-9930;(c) F.C. Liu, Y.F. Zeng, J.P. Zhao, et al., An unusual 1D manganese azido complex with novel EO/EO/EO/EE coordination mode: synthesis, structure, and magnetic properties, Inorg. Chem. 46 (2007) 1520-1522. |
| [9] | (a) X.J. Li, X.F. Guo, X.L. Weng, S. Lin, Two novel 2D cadmium(II) MOFs based on flexible bis(imidazolyl) and zwitterionic dicarboxylate ligands, CrystEngComm 14 (2012) 1412-1418;(b) Y.Q. Wang, Q. Yue, Y. Qi, et al., Manganese(II), iron(II), and mixed-metal metal-organic frameworks based on chains with mixed carboxylate and azide bridges:magnetic couplingand slowrelaxation, Inorg. Chem. 52(2013) 4259-4268. |
| [10] | (a) S.D. Han, W.C. Song, J.P. Zhao, et al., Synthesis and ferrimagnetic properties of an unprecedented polynuclear cobalt complex composed of [Co24] macrocycles, Chem. Commun. 49 (2013) 871-873;(b) D. Foguet-Albiol, K.A. Abboud, G. Christou, High-nuclearity homometallic iron and nickel clusters: Fe22 and Ni24 complexes from the use of N-methyldiethanolamine, Chem. Commun. (2005) 4282-4284;(c) L. Han, H. Valle, X.H. Bu, Homochiral coordination polymer with infinite double-stranded helices, Inorg. Chem. 46 (2007) 1511-1513;(d) X.L. Hong, J. Bai, Y. Song, Y.Z. Li, Y. Pan, Luminescent open-framework antiferromagnet-hydrothermal syntheses, structures, and luminescent and magnetic properties of two novel coordination polymers: [Zn(pdoa)(bipy)]n and {[Mn(pdoa)(bipy)](bipy)}n [pdoa=2,20-(1,3-phenylenedioxy)bis(acetate);bipy=4,40-bipyridine], Eur. J. Inorg. Chem. 45 (2006) 3659-3666. |
| [11] | F.K. Zheng, A.Q. Wu, Y. Li, G.C. Guo, J.S. Huang, Synthesis and structural characterization of a new cadmium (II) complex with a double betaine, Chin. J. Struct. Chem. 8 (2005) 940-944. |
| [12] | A.X.S. Bruker, SAINT Software Reference Manual, Bruker Analytical X-ray Instruments Inc., Madison, WI, 1998. |
| [13] | G.M. Sheldrick, SADABS, Siemens Area Detector Absorption Corrected Software, University of Göttingen, Germany, 1996. |
| [14] | G.M. Sheldrick, A short history of SHELX, Acta Crystallogr. A 64 (2008) 112-122. |
| [15] | Y.Q. Wang, Q.X. Jia, K. Wang, A.L. Cheng, E.Q. Gao, Diverse manganese(II) coordination polymers with mixed azide and zwitterionic dicarboxylate ligands: structure and magnetic properties, Inorg. Chem. 49 (2010) 1551-1560. |