b Department of Chemical and Biomolecular Engineering, University of California at Los Angeles, Los Angeles, CA 90095, USA;
c Materials Research Department, Toyota Motor Engineering and Manufacturing North America, Inc., 1555 Woodridge Avenue, Ann Arbor, MI 48105, USA;
d Institute of Chemistry, Chinese Academy of Sciences, Beijing 100190, China
Lead telluride and its alloys are highly potential in the field of solid-state thermoelectric (TE) cooling and electrical power generation due to their narrow band gaps and face-centered cubic structure [1, 2, 3]. These materials are known as the most promising candidate for TE devices in the temperature range of 500-1000 K, which makes them particularly suitable for recovering electrical power from heat energy of automotive exhaust systems,solar energy converters,and combustible solid waste. The low-cost conversion process of TE materials is expected to play an important role in solving the energy and environmental problems in the future. PbTe-based materials have been extensively used in power supplies for space exploration and military purposes. However, their current application areas are rather limited because of the relatively low energy conversion efficiency. In order to improve the TE performance,tremendous efforts have been made in recent years to improve the figure of merit (ZT) of thermoelectric materials.
It is reported that high TE performance can be achieved through minimization of the thermal conductivity. For example, nanocomposites in bulk form have compositional fluctuations at the large number of interfaces,resulting in a reduced phonon propagation and thermal conductivity [2, 4, 5]. The possibility of improving the ZT value by alloying PbTe with SnTe was also proposed since these pseudobinary solid solutions generally have a more favorable ratio of electrical conductivity to thermal conductivity than either of the components [6]. Recent investigation revealed that the TE efficiency of PbTe can be enhanced by distortion of the electronic density of states [7]. Moreover, theoretical calculations indicate that ZT values can be significantly increased by quantum confinement effect in low dimensional nanostructured materials [8, 9],which is verified by experimental studies on the systems of nanolayered quantum-well superlattices [10],nanowires [11, 12, 13, 14],and quantum-dot superlattice thin films [1, 15, 16]. Therefore,synthesis of nanostructured PbTe and its alloys has attracted much attention in recent years [17, 18, 19, 20, 21, 22, 23, 24, 25, 26, 27]. Among these materials,PbTe nanocrystals are expected to yield high ZT because of the tunable quantum effects by controlling the particle size and shape. It has been demonstrated that hotinjection is an effective way to prepare PbTe nanocrystals [23, 24, 25, 26, 27]. In order to obtain uniform and monodispersed PbTe nanocrystals, this procedure usually involves the usage of oleic acid as ligands. However,it is hard to remove the ligands completely and the presence of the residual organic component may result in brittle devices with deteriorated electric conductivity. Thus,how to find an effective way to remove the ligands thoroughly becomes critical to achieve desirable thermoelectric performance. Previous work has proved that hydrazine molecules can replace bulky oleic acid at the surface of the nanocrystals,reducing the interparticle spacing and doping the solid [23]. However,in our experiment,some of the organic molecules still remained in the samples after treatment. Recently,reduction of carboxyl groupviaa super-hydride reaction with lithium triethylborohydride has proved to be an effective way to remove the organic ligands on the surface of TiO2 nanocrystals [28, 29]. After reduction,the carboxyl group was transformed to hydroxyl group,losing the coordination capacity with the nanocrystals. In this paper,PbTe nanocrystals and its alloys with SnTe were synthesized by a solvothermal approach. This approach enables mass and economical synthesis of PbTe-based hybrid nanocrystals with designed shape,chemical composition and narrow size distribution. Moreover,ligands-free samples with increased electrical conductivity were obtained by pretreatment with a super-hydride solution of lithium triethylborohydride and trimethylchlorosilane. The purpose of the addition of trimethylchlorosilane is to promote the extent of the reduction,making it possible to fabricate fully dense and robust thermoelectric devices by employing these materials as building blocks. 2. Experimental
2.1. Synthesis of PbTe/SnTe hybrid nanocrystals
PbTe/SnTe hybrid nanocrystals were prepared by solvothermal strategy using toluene as solvent and oleic acid as ligands. Typically,tributylphosphine telluride (TBP-Te) was first prepared as Te-source by dissolving 2.552 g of tellurium powder (20 mmol) in 7 mL of TBP with ultrasonication. 3.793 g of lead acetate trihydrate was added to 6.02 g of oleic acid and the mixture was heated under nitrogen at 100°C for 1 h to promote the formation of lead oleate. Certain amount of lead oleate and bis[bis(trimethylsilyl)amino]tin(II) was then dissolved in 40 mL of toluene and transferred to a Teflon-lined stainless steel autoclave. Subsequently,TBP-Te was diluted with 40 mL of toluene and added to the above solution. The molar ratio of lead oleate:bis[bis(trimethylsilyl)amino]tin(II):TBP-Te was x:1-x:2 (0≤x≤1). The autoclave was sealed and maintained at 150°C for 140 min,then cooled down to room temperature with a water bath. To isolate and purify the nanocrystals,equal volume of acetone was added to the crude solution followed by centrifugation. The product was additionally purified twice by dispersing them in hexane and precipitating with acetone. Finally,the particles were dissolved in hexane forming stable colloidal solutions. In order to remove the organic ligands, the nanocrystals were precipitated by acetone followed by immersion in a super-hydride solution,1 mol/L lithium triethylborohydride (LiBEt3H) in THF under nitrogen and sonication for 30 min,then centrifuged and washed with THF. This procedure was repeated for three times. In order to promote the extent of the reaction,trimethylchlorosilane (Me3SiCl,equal molar ratio to LiBEt3H) was also added into the super-hydride solution. 2.2. Characterization of PbTe/SnTe hybrid nanocrystals
Transmission electron microscopy (TEM) images for morphology characterization were obtained using a JEOL 100CX electron
microscope operated at an accelerating voltage of 100 kV. Highresolution TEM (HRTEM) image and elemental composition
calculation from the energy-dispersive X-ray spectroscopy (EDX)
were taken on a JEOL 2011 electron microscope with an
accelerating voltage of 200 kV. Samples for TEM analysis were
prepared by depositing a drop of dilute solution in hexane on a 300
mesh carbon-coated copper grid. SEM observation was performed
on a JEOL JSM-6700F scanning electron microscope. Wide-angle
powder X-ray diffraction (XRD) measurement was carried out on a
Philips Xpert X-ray diffractometer using Cu Ka radiation
( = 0.1542 nm). The electrical conductivity was measured by a
Jandel four-point probe equipped with Keithley 6221/2182A
current generator and voltmeter. Fourier transform infrared
spectrometry (FTIR) was carried out on a Jasco FT/IR-420
spectrometer. The thermal decomposition behavior of the organic
ligands was monitored using a Mettler Toledo TGA/SDTA851
analyzer.
3. Results and discussion
= 0.1542 nm). The electrical conductivity was measured by a
Jandel four-point probe equipped with Keithley 6221/2182A
current generator and voltmeter. Fourier transform infrared
spectrometry (FTIR) was carried out on a Jasco FT/IR-420
spectrometer. The thermal decomposition behavior of the organic
ligands was monitored using a Mettler Toledo TGA/SDTA851
analyzer.
3. Results and discussion
PbxSn1.xTe nanocrystals with different chemical composition
were synthesized in this work by controlling the amount of lead
oleate and bis[bis(trimethylsilyl)amino]tin(II) via solvothermal
approach. When the molar ratio of lead oleate to bis[bis(trimethylsilyl)amino]tin(II) varied from 1:0,0.9:0.1,to 0.8:0.2,the
corresponding elemental composition of the nanocrystals calculated from the EDX results proved to be PbTe,Pb0.82Sn0.18Te and
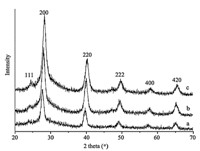
Pb0.71Sn0.29Te,respectively (Fig. 1). All of the as-synthesized
PbxSn1-xTe nanocrystals have uniform,nearly spherical morphologies with an average diameter of 8 nm (Fig. 2). These nanoparticles with a narrow size distribution can self-assemble into
ordered superlattice patterns on a substrate of copper grid or
silicon wafer upon slow evaporation of concentrated hexane
solutions. The size of the nanocrystals can be controlled by adjusting the reaction time,growth temperature,and the ratio of
oleic acid to lead acetate. Generally,extended reaction time and
high temperature result in increased particle size. In addition,low
ratio of oleic acid to lead acetate is favorable for the synthesis of
small nanoparticles. For low oleic acid concentrations,the fast
nucleation rate leads to large amount of nuclei,thus forming small
nanocrystals. In our experiment,the optimal molar ratio of oleic
acid to lead acetate is between 2.1 and 2.5. The ability to tune the
size of the nanocrystals potentially provides the possibility of
tuning the quantum effect,which is critical to achieve an enhanced
thermoelectric figure of merit. HRTEM image of PbTe nanocrystal
(Fig. 2d) shows the material has a crystalline structure with an
adjacent lattice-fringe distance of ca. 3.2 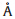 ,which is consistent
with the (2 0 0) interplanar distance (3.23
,which is consistent
with the (2 0 0) interplanar distance (3.23 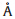 ) of the face centered
cubic (FCC) PbTe.
) of the face centered
cubic (FCC) PbTe.

|
Download:
|
| Fig. 1. Energy-dispersive X-ray spectroscopy of PbxSn1-xTe nanocrystals with different chemical composition: (a) PbTe; (b) Pb0.82Sn0.18Te; (c) Pb0.71Sn0.29Te | |

|
Download:
|
| Fig. 2. TEM images of as-synthesized PbxSn1-xTe nanocrystals with different chemical composition: (a) PbTe; (b) Pb0.82Sn0.18Te; (c) Pb0.71Sn0.29Te. (d) HRTEM image of an individual PbTe nanocrystal. | |
The crystallinity of the nanocrystals was also confirmed by XRD
measurement. As shown in Fig. 3,the obtained nanocrystals have
well-defined cubic rock-salt crystal structure withFm3mspace
group. The diffraction peaks are broader than those of the bulk
sample,in accordance with their smaller crystalline domains. With
more lead atoms replaced by tin,the diffraction peaks tend to shift
toward higher angle,indicating a decrease in the unit cell
parameter (a). Indeed,as a result of the smaller diameter of tin
atoms,theavalue changed from 6.459 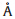 for PbTe to 6.391
for PbTe to 6.391 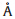 and
6.320
and
6.320 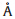 for Pb0.82Sn0.18Te and Pb0.71Sn0.29Te,respectively. The
average crystal size of the samples was estimated as around 8.4 nm
by applying Scherrer formula to the broadening of the XRD
reflections,which is consistent with the information obtained from
TEM observation.
for Pb0.82Sn0.18Te and Pb0.71Sn0.29Te,respectively. The
average crystal size of the samples was estimated as around 8.4 nm
by applying Scherrer formula to the broadening of the XRD
reflections,which is consistent with the information obtained from
TEM observation.
|
Download:
|
| Fig. 3. -ray diffraction patterns of PbxSn1-xTe nanocrystals with different composition: (a) PbTe; (b) Pb0.82Sn0.18Te; (c) Pb0.71Sn0.29Te. | |
In order to evaluate the electrical transport properties of PbxSn1.xTe nanocrystals,the obtained powders were consolidated by spark plasma sintering (SPS) method at 400°C for 5 min under vacuum. The as-synthesized samples after consolidation showed very poor electrical conductivities of about 10-13S/cm,due to the difficulty of charge transport through the large interparticle separations occupied by the insulating capping ligands of oleic acid. The presence of the organic ligands may also prevent the formation of effective metallic bonding between the nanoparticles, resulting in brittle sintered solids with low density. Therefore, ligands-free samples will be critical to achieve dense and robust devices with high electrical conductivity for practical applications. To solve this problem,herein we employed a reduction method to remove the oleic acid with a super-hydride solution,1 mol/L lithium triethylborohydride (LiBEt3H) in THF. In order to promote the extent of the reaction,trimethylchlorosilane was also added into the superhydride solution. Fig. 4a shows a TEM image of PbTe nanocrystals after treatment with LiBEt3H/Me3SiCl. Compared with the sample in Fig. 2a,the interparticle spacing after treatment was decreased and some of the nanocrystals aggregated due to the removal of the organic ligands,which is helpful to facilitate the tunneling between the nanocrystal. SEM image of PbTe nanocrystals after treatment with LiBEt3H/Me3SiCl and consolidation by SPS (Fig. 4b) also demonstrates that some nanocrystals were tunneled together and larger particles with a size ranging from 20 nm to 100 nm were formed. Therefore,the electrical conductivity of PbTe nanocrystals was greatly increased by 15 orders of magnitude,which was measured to be around 160 S/cm. In addition,to study the effect of sintering temperature on the structure of the consolidated samples, the nanocrystals were sintered at different temperature. The results showed that at lower temperature,for example 300°C,the obtained sample was very fragile. While at higher temperature of 500°C, severe fusion of the nanocrystals was found and the nanostructure was destroyed. Therefore,the optimized sintering temperature might be around 400°C in this paper.
|
Download:
|
| Fig. 4. (a) TEM image of PbTe nanocrystals after treatment with LiBEt3H/Me3SiCl; (b) SEM image of PbTe nanocrystals after treatment with LiBEt3H/Me3SiCl and consolidation by spark plasma sintering (SPS) at 400°C for 5 min under vacuum | |
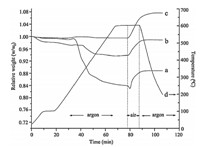
TGA experiments were conducted to investigate the efficiency of the above-mentioned methods,and the curves are shown in Fig. 5,taking PbTe nanocrystals as an example. The samples were first heated to 100°C under argon at a heating rate of 10°C/min and held for 10 min to remove the residual solvent. The temperature was then increased to 600°C at the same heating rate and held for 10 min. Subsequently,the samples were exposed to air for 10 min before temperature was decreased to room temperature in argon (Fig. 5d). The as-prepared sample before treatment with super-hydride shows that 17% of the organic component was removed during heating,followed by another 1% weight loss when exposed to air,due to the combustion of the carbonized organic ligands (Fig. 5a). For the sample after treatment with LiBEt3H (Fig. 5b),about 6.4% of the organic component was detected. While for the samples after chemical treatment with LiBEt3H/Me3SiCl (Fig. 5c),there is no weight loss,indicating no organic ligands and derived carbon was in the sample,which demonstrates that the organic ligands were totally removed by the combination of LiBEt3H and Me3SiCl. This method is promising for the fabrication of dense and robust devices. The weight increase of the samples after exposure to air is resulted from the oxidation of PbTe nanocrystals.
|
Download:
|
| Fig. 5. TGA curves of (a) as-prepared PbTe nanocrystals; (b) PbTe nanocrystals after treatment with LiBEt3H; (c) PbTe nanocrystals after treatment with LiBEt3H/Me3SiCl. (d) Curve of temperature change with time | |
The efficiency of LiBEt3H/Me3SiCl in the removal of organic ligands was further confirmed by FTIR spectra. As shown in Fig. 6, the strong absorption (Fig. 6a) for the as-synthesized PbTe nanocrystals at 1735 cm-1 is assigned to the stretching vibration of the carboxylate groups. The two sharp peaks at 2847 and 2923 cm-1 are attributed to the -CH stretching vibrations of oleic acid. After treatment with the super-hydride solution (Fig. 6b), these absorptions become much weaker. This reveals that a little part of the organic ligands was left in the sample after treatment with LiBEt3H. For the samples washed by LiBEt3H/Me3SiCl,the organic absorptions disappeared completely (Fig. 6c),indicating oleic acid was totally removed,which is consistent with the TGA results. In addition,the removal of the oleic acid for another two samples,Pb0.82Sn0.18Te and Pb0.71Sn0.29Te,was also performed. Similar TGA and FTIR results were obtained,indicating the generality of the reduction method employed in this work. The validity of LiBEt3H/Me3SiCl in the removal of the organic ligands can possibly be explained by the following mechanism. As shown in Eqs. (1) and (2),during the reduction of oleic acid,LiBEt3H was first oxidized to LiBEt3OH,and then further reacted with the added Me3SiCl,resulting in the generation of LiCl. Therefore,the addition of Me3SiCl makes the equilibrium of Eq. (1) moves to the right,thus promoting the extent of the reduction of oleic acid.
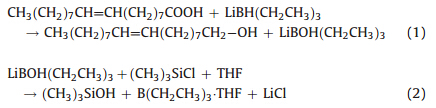

|
Download:
|
| Fig. 6. FTIR spectra of (a) as-prepared PbTe nanocrystals; (b) PbTe nanocrystals after treatment with LiBEt3H; (c) PbTe nanocrystals after treatment with LiBEt3H/Me3SiCl. | |
In summary,we have developed an effective solvothermal approach toward the synthesis of PbxSn1.xTe nanocrystals with designed chemical composition and narrow size distribution. The organic ligands were completely removed by pretreatment with a super-hydride solution of lithium triethylborohydride and trimethylchlorosilane,making it possible to fabricate fully dense and robust thermoelectric devices with increased electrical conductivity. Acknowledgments
This work was supported by the National Natural Science Foundation of China (No. 51173074),the Key Project of Chinese Ministry of Education (No. 212099),the Promotive Research Fund for Young and Middle-aged Scientists of Shandong Province (No. BS2012CL010),and Toyota Motor Engineering & Manufacturing North America (TEMA) Inc.
| [1] | T.C. Harman, P.J. Taylor, M.P. Walsh, B.E. LaForge, Quantum dot superlattice thermoelectric materials and devices, Science 297 (2002) 2229-2232. |
| [2] | K.F. Hsu, S. Loo, F. Guo, et al., Cubic AgPbmSbTe2+m: bulk thermoelectric materials with high figure of merit, Science 303 (2004) 818-821. |
| [3] | Y.M. Lin, M.S. Dresselhaus, Thermoelectric properties of superlattice nanowires, Phys. Rev. B 68 (2003) 075304. |
| [4] | J. Androulakis, K.F. Hsu, R. Pcionek, et al., Nanostructuring and high thermoelectric efficiency in p-type Ag(Pb1-ySny)mSbTe2+m, Adv. Mater. 18 (2006) 1170-1173. |
| [5] | M. Zhou, J. Li, T. Kita, Nanostructured AgPbmSbTem+2 system bulk materialswith enhanced thermoelectric performance, J. Am. Chem. Soc. 130 (2008) 4527-4532. |
| [6] | V. Damodara Das, C. Bahulayan, Variation of electrical transport properties and thermoelectric figure of merit with thickness in 1% excess Te-doped Pb0.2Sn0.8Te thin films, Semicond. Sci. Technol. 10 (1995) 1638-1644. |
| [7] | J.P. Heremans, V. Jovovic, E.S. Toberer, et al., Enhancement of thermoelectric efficiency in PbTe by distortion of the electronic density of states, Science 321 (2008) 554-557. |
| [8] | L.D. Hicks, M.S. Dresselhaus, Effect of quantuμ-well structures on the thermoelectric figure of merit, Phys. Rev. B 47 (1993) 12727-12731. |
| [9] | L.D. Hicks, M.S. Dresselhaus, Thermoelectric figure of merit of a one-dimensional conductor, Phys. Rev. B 47 (1993) 16631-16634. |
| [10] | R. Venkatasubramanian, E. Siivola, T. Colpitts, B. O'Quinn, Thin-film thermoelectric devices with high rooμ-temperature figures of merit, Nature 413 (2001) 597-602. |
| [11] | M.S. Sander, A.L. Prieto, R. Gronsky, T. Sands, A.M. Stacy, Fabrication of highdensity, high aspect ratio, large-area bismuth telluride nanowire arrays by electrodeposition into porous anodic alumina templates, Adv. Mater. 14 (2002) 665-667. |
| [12] | M. Martin-González, A.L. Prieto, R. Gronsky, T. Sands, A.M. Stacy, High-density 40 nm diameter Sb-rich Bi2-xSbxTe3 nanowire arrays, Adv. Mater. 15 (2003) 1003-1006. |
| [13] | A.I. Boukai, Y. Bunimovich, J. Tahir-Kheli, et al., Silicon nanowires as efficient thermoelectric materials, Nature 451 (2008) 168-171. |
| [14] | A.I. Hochbaum, R. Chen, R.D. Delgado, et al., Enhanced thermoelectric performance of rough silicon nanowires, Nature 451 (2008) 163-167. |
| [15] | T.C. Harman, D.L. Spears, M.J. Manfra, High thermoelectric figures of merit in PbTe quantum wells, J. Electron. Mater. 25 (1996) 1121-1127. |
| [16] | H. Beyer, J. Nurnus, H. Bottner, et al., PbTe based superlattice structures with high thermoelectric efficiency, Appl. Phys. Lett. 80 (2002) 1216-1218. |
| [17] | W. Wang, B. Poudel, D. Wang, Z. Ren, Synthesis of PbTe nanoboxes using a solvothermal technique, Adv. Mater. 17 (2005) 2110-2114. |
| [18] | X.F. Qiu, Y.B. Lou, A.C.S. Samia, et al., PbTe nanorods by sonoelectrochemistry, Angew. Chem. Int. Ed. 44 (2005) 5855-5857. |
| [19] | W.F. Liu, W. Cai, L.Z. Yao, Electrochemical deposition of well-ordered singlecrystal PbTe nanowire arrays, Chem. Lett. 36 (2007) 1362-1363. |
| [20] | M. Fardy, A.I. Hochbaum, J. Goldberger, M. Zhang, P. Yang, Synthesis and thermoelectrical characterization of lead chalcogenide nanowires, Adv. Mater. 19 (2007) 3047-3051. |
| [21] | W.X. Zhang, L. Zhang, Y.W. Cheng, et al., Synthesis of nanocrystalline lead chalcogenides PbE (E=S, Se, or Te) from alkaline aqueous solutions, Mater. Res. Bull. 35 (2000) 2009-2015. |
| [22] | D. Wang, C. Song, X. Fu, Z. Hu, Surfactant-assisted synthesis of cube-shaped PbTe and PbSe nanocrystals, J. Dispers. Sci. Technol. 28 (2007) 1197-1200. |
| [23] | J.J. Urban, D.V. Talapin, E.V. Shevchenko, C.B. Murray, Self-assembly of PbTe quantum dots into nanocrystal superlattices and glassy films, J. Am. Chem. Soc. 128 (2006) 3248-3255. |
| [24] | W.G. Lu, J.Y. Fang, K.L. Stokes, J. Lin, Shape evolution and self assembly of monodisperse PbTe nanocrystals, J. Am. Chem. Soc. 126 (2004) 11798-11799. |
| [25] | T. Mokari, M.J. Zhang, P.D. Yang, Shape, size, and assembly control of PbTe nanocrystals, J. Am. Chem. Soc. 129 (2007) 9864-9865. |
| [26] | Z.H. Lin, M.Q. Wang, L.Z. Wei, et al., PbTe colloidal nanocrystals: synthesis, mechanism and infrared optical characteristics, J. Alloys Compd. 509 (2011) 5047-5049. |
| [27] | Z.H. Lin, M.Q. Wang, L.Y. Zhang, et al., Equilibrium self-assembly of close-packed ordered PbTe nanocrystal thin film and near-infrared photoconductive detector, J. Mater. Chem. 22 (2012) 9082-9085. |
| [28] | J. Joo, S.G. Kwon, T. Yu, et al., Large-scale synthesis of TiO2 nanorods via nonhydrolytic sol-gel ester elimination reaction and their application to photocatalytic inactivation of E. coli, J. Phys. Chem. B 109 (2005) 15297-15302. |
| [29] | C. Carlucci, H. Xu, B.F. Scremin, et al., Selective synthesis of TiO2 nanocrystals with morphology control with the microwave-solvothermal method, CrystEngComm 16 (2014) 1817-1824. |




