b Department of Chemistry, College of Chemistry and Chemical Engineering, Xiamen University, Xiamen 361005, China
Thiolate-protected noble metal nanoclusters have been attracting increasing research interests owing to their potential applications in bio-labeling and sensing,drug delivery and medical therapy,molecular recognition and molecular electronics,and catalysis [1, 2, 3, 4, 5, 6, 7]. Many applications of thiolated noble metal nanoclusters are beneficial from their excellent stability. Different from classical nanoparticles,the molecular structures of atomically precise noble metal nanoclusters are readily determined by X-ray diffraction techniques [8, 9, 10, 11, 12]. The solutions of thiolated noble metal nanoclusters have made it possible to use powerful computational techniques to gain insights into the origins of their chemical stabilities [13]. The electronic shell closing based on the superatom theory has successfully been applied to explain the exceptional stability of many noble metal nanoclusters [14, 15, 16].
In the superatom theory,the effects of surface ligands and counterions on the stability of noble metal nanoclusters were mainly reflected on whether they withdrew or provided extra charges on the clusters [13, 17]. In the term of electronic shell closing,the influences of detailed structural parameters,for example,different substitutions on surface ligands or bulkiness of counterions,were considered unessential to the overall stability of a certain cluster. In many theoretical explanations of optical absorption spectra of metal nanoclusters,the surface thiolates were even simplified as HS- or CH3S- [18, 19]. However,several experimental studies have already revealed substantial impacts of surface ligands on the stabilities and even properties of noble metal clusters [20, 21, 22]. For example,Murray and coworkers demonstrated the obvious effects of thiol ligands on the redox properties of thiol-stabilized Au38(SPhX)24 clusters (where X = NO2,Br,H,CH3,and OCH3) [20]. Changing the surface ligands from thiolate to selenolate was found to increase the stability of Au nanoclusters [22]. With the experimental observations on the ligand effects on Au nanoclusters,more and more theoretical calculations have been recently performed to understand how surface ligands influence on the electronic structure and stability of metal nanoclusters. Han and co-workers [23] have studied by DFT calculations on the ligand effects on the stability of thiolstabilized gold nanoclusters,Aum(SR)n. Besides the ligand effects, the effects of metal doping on the electronic structures and stabilities of metal nanoclusters were also studied by both experimental and theoretical means [19, 24, 25, 26]. However,experimental studies on the effects of detailed structural parameters on the stabilities of thiolated metal nanoclusters whose structures were crystallographically confirmed have not been reported in the literature.
We now demonstrate how fine tuning of surface ligands and counter cations can significantly affect the stability of thiolated Ag44 and Au12Ag32 nanoclusters. Series of [M12Ag32(SR)30]4- (M = Ag,Au) clusters with -SPhF2,-SPhCF3 or -SPhF as surface ligands,and PPh4 + or [(PPh3)2N]+ as counter cations were prepared and structurally characterized by X-ray diffraction for the stability studies. Our systematic investigations revealed that the nanoclusters with -SPhF2 as the surface ligands exhibited much better stabilities than those with -SPhCF3 or -SPhF. Changing the counter cations from PPh4 + to more bulky [(PPh3)2N]+ significantly reduced the clusters’ stabilities. Compared to [Ag44(SR)30]4- clusters with the same surface ligands and counter cations,[Au12Ag32(SR)30]4- displayed much better stabilities.
2. Experimental 2.1. Synthesis of (PPh4)4[M12Ag32(SR)30] (M = Ag,Au and SR = SPhF2, SPhCF3,SPhF)The (PPh4)4[M12Ag32(SR)30] samples were synthesized following the method that we have recently reported [11]. In a typical synthesis of the [M12Ag32(SR)30]4- nanoclusters,the metal (e.g.,Ag, Au) precursors were chemically reduced by an aqueous solution of NaBH4 in the presence of thiol and PPh4Br in a mixed solvent of CH2Cl2 and CH3OH at 0 ℃ in an ice bath. Pure (PPh4)4 [M12Ag32(SR)30] samples were obtained in the form of single crystals by layering hexane into the CH2Cl2 solutions of clusters at 4 ℃.
2.2. Synthesis of [(PPh3)2N]4[M12Ag32(SR)30] (M = Ag,Au and SR = SPhF2,SPhCF3)The [(PPh3)2N]4[M12Ag32(SR)30] samples were prepared by the same method as (PPh4)4[M12Ag32(SR)30] except that bis-(triphenylphosphoranylidene) ammonium chloride,(PPh3)2NCl,instead of PPh4Br was used as the salt to supply counter cations to balance the charge from [M12Ag32(SR)30]4- clusters.
2.3. Stability evaluation by UV-vis spectroscopyPure crystals containing specific [M12Ag32(SR)30]4- clusters and counter cations were dissolved in DMF for the stability studies. The solutions were heated in closed glass vessels in air at 80 ℃. Their UV-vis spectra were monitored with the heating time to evaluate the stabilities of the clusters.
2.4. TEM characterizations of treated [Ag44(SPhF2)30]4- clustersTEM studies were performed on a TECNAI F-30 transmission electron microscope operating at 300 kV. The samples were prepared by dropping the DMF solutions of (PPh4)4[Ag44(SR)30] that were heated in air at 80 ℃ for 3 and 12 h onto 300-mesh carbon-coated copper grids and immediately evaporating the solvent.
3. Results and discussionOwing to their well-defined molecular structures,atomicprecise nanoclusters readily serve as an unique class of nanoparticles for evaluating the influences of detailed structural parameters on the overall stability of nanoparticles,which is hardly investigated by other nanoparticulate systems. In order to evaluate the effects of surface ligands,counter cations and metal compositions on the stability of metal nanoparticles. In this work, the following three series of [M12Ag32(SR)30]4- nanoclusters were first synthesized following our recently reported method [11]: (1) Ag44 clusters stabilized by different thiolates (i.e.,SPhF2,SPhCF3, SPhF); (2) Au12Ag32 clusters (with M = Au) capped by the same thiolate as their Ag44 counterparts; (3) Ag44 and Au12Ag32 clusters stabilized by the same thiolate but with charges balanced by different counter cations {i.e.,PPh4 +,[(PPh3)2N]+}.
As determined by X-ray crystallography (see Supporting information for detailed crystallographic data),all of the clusters have the same total structure as those (PPh4)4[M12Ag32(SR)30] (SR = SPhF2,SPhCF3,SPhF) clusters [11]. As shown in Fig. 1a,each [M12Ag32(SR)30]4- cluster can be described as a Keplerate twoshell M12@Ag20 core with an icosahedral M12 unit encapsulated in a dodecahedral Ag20 shell (Fig. 1b). The core is protected by six Ag2(SR)5 units on its surface to figure the overall structure of the clusters. Each [Ag44(SR)30]4- cluster has a charge of-4 balanced by four PPh4 + or [(PPh3)2N]+ cations (Fig. 1c). As shown in their crystal packings (Figs. S1-S8 in Supporting information),the rigid counter cations are occupying the intercluster space and helping to prevent free rotation of clusters in solid state. Owing to the rather large size of the clusters,the extracluster spaces were calculated to be more than 35% in all the obtained single crystals containing [M12Ag32(SR)30]4- clusters.

|
Download:
|
| Fig. 1.(a) The overall structure of the [M12Ag32(SR)30]4- (M = Au,Ag) cluster. All hydrogen and fluorine atoms are omitted for clarity. (b) The two-shell M12@Ag20 core of the cluster. (c) The structures counter cations,PPh4 + and [(PPh3)2N]+. Color legend: gold sphere,M; green sphere,Ag; yellow sphere,S; pink sphere,P; gray sphere and stick,C. (d) UV-vis absorption spectra of [Ag44(SR)30]4- with different surface ligands and counter cations. | |
All [M12Ag32(SR)30]4- clusters exhibited broad multiband optical absorptions in solutions. As detailed in Fig. 1d,the [Ag44(SR)30]4- clusters displayed similar absorption features with four major peaks at 410,480,535,and 640 nm. The absorption bands of the clusters did neither vary with the surface thiolate ligands nor with the counter cations. The [Ag44(SR)30]4- clusters were stable in the DMF solution at room temperature for months with no obvious change in their optical spectra,which was explained by an 18-electron superatom shell closure in the metal core [11]. However,the clusters were not stable upon heating in air at 80 ℃. Such a thermal instability made it possible for us to effectively evaluate the effects of surface ligands and counter cations on the overall stability of the clusters by monitoring the UV-vis spectra of their DMF solutions with the heating time.
Surprisingly,as illustrated in Fig. 2,the [Ag44(SR)30]4- clusters displayed distinct stabilities that were highly dependent on their surface thiolate ligands. When PPh4 + was used as the counter cations,[Ag44(SPhF2)30]4- exhibited far better stability than [Ag44(SPhCF3)30]4- and [Ag44(SPhF)30]4-. The [Ag44(SPhF2)30]4- clusters in DMF were stable for more than 5 h in air at 80 ℃ (Fig. 2a). (PPh4)4[Ag44(SPhF2)30] in DMF started to degrade beyond 5 h with the absorption peaks broadened and their intensities decreased gradually with time. After being heated in air at 80 ℃ for 12 h,(PPh4)4[Ag44(SPhF2)30] in DMF changed its color from rose red to yellow. All the absorption peaks of the clusters disappeared and a new sharp peak at 430 nm appeared. Such an absorption feature indicated that the nanoclusters were aggregated to form larger Ag nanoparticles. Indeed,as illustrated in Fig. 2b,our TEM measurements revealed that nanoparticles with size much larger than that of original nanoclusters were formed at 12 h,while no obvious formation of large Ag nanoparticles was observed at 3 h. Compared to [Ag44(SPhF2)30]4-,[Ag44(SPhCF3)30]4- and [Ag44(SPhF)30]4- clusters exhibited much poor stabilities when heated at 80 ℃ in air. As shown in Fig. 2c and d, (PPh4)4[Ag44(SPhCF3)30] in DMF started to degrade after 40 min, and (PPh4)4[Ag44(SPhF)30] started to degrade even in less than 10 min. Similar to (PPh4)4[Ag44(SPhF2)30],(PPh4)4[Ag44(SPhCF3)30] and (PPh4)4[Ag44(SPhF)30] were eventually transformed into larger Ag nanoparticles with only one sharp absorption peak at 430 nm. It took only 90 and 70 min for (PPh4)4[Ag44(SPhCF3)30] and (PPh4)4[Ag44(SPhF)30],respectively,to be fully aggregated into large Ag nanoparticles. Such a significant effect of surface ligands on the stability of [Ag44(SR)30]4- is unexpected since all the three clusters have 18-electron superatom shell closure in their metal cores.

|
Download:
|
| Fig. 2. UV-vis spectra of DMF solutions of (PPh4)4[Ag44(SR)30] heated in air at 80 ℃ for different time: SR = SPhF2 (a),SPhCF3 (c),and SPhF (d). (b) TEM images of the DMF solution of (PPh4)4[Ag44(SPhF2)30] that were heated in air at 80 ℃ for 3 and 12 h. | |
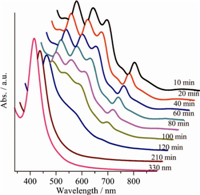
It was even more inconceivable that the stabilities of [Ag44(SR)30]4- clusters were also highly determined by their counter cations. The same clusters exhibited significantly different stabilities when their counter cations were changed. In the case of [Ag44(SPhF2)30]4-,changing their counter cations from PPh4 + to more bulky [(PPh3)2N]+ cations reduced the stability of the clusters dramatically. As shown in Fig. 3,when heated in air at 80 ℃, [(PPh3)2N]4[Ag44(SPhF2)30] in DMF started to degrade beyond 1 h. In comparison,(PPh4)4[Ag44(SPhF2)30] was stable for more than 5 h under the same conditions. The dramatic influence of counter cations on the stability of the [Ag44(SPhF2)30]4- in solutions clearly indicated that those counter cations could be still somewhat associated with the anionic clusters when dissolved in solutions. The counter cations surrounding [Ag44(SPhF2)30]4- would help to protect them from aggregation. The poorer stability of [(PPh3)2N]4[Ag44(SPhF2)30] might be explained by the rather bulky feature of [(PPh3)2N]+ cations,making them less effective to protect the clusters from thermal aggregation.
|
Download:
|
| Fig. 3. UV-vis spectra of the DMF solution of [(PPh3)2N]4[Ag44(SPhF2)30] heated in air at 80 ℃ for different time. | |
Besides the surface ligands and charge-balancing counter cations,the composition of the metal core was also found essential to the overall stability of the [M12Ag32(SR)30]4- clusters. Substituting the core Ag12 unit with Au12 significantly enhanced the stability of the clusters. Experimentally,such a compositional substitution was readily achieved by introducing Au precursors together with Ag salts,and confirmed by single crystal analysis. The incorporation of Au dramatically altered both the optical properties and stability of the clusters. As shown in Fig. 4,the [Au12Ag32(SPhF2)30]4- displayed only two pronounced peaks at 390 and 490 nm with three weak shoulder peaks at 575,620 and 730 nm. When the surface capping ligands and counter cations were same,the [Au12Ag32(SR)30]4- clusters were much more stable than [Ag44(SR)30]4-. While (PPh4)4[Ag44(SPhF2)30] was stable in DMF only for 5 h in air at 80 ℃,(PPh4)4[Au12Ag32(SPhF2)30] dissolved in DMF was stable under the same conditions for more than 8 days (Fig. 4a)

|
Download:
|
| Fig. 4. UV-vis spectra of DMF solutions of (a) (PPh4)4[Au12Ag32(SPhF2)30]; (b) (PPh4)4[Au12Ag32(SPhCF3)30]; (c) (PPh4)4[Au12Ag32(SPhF)30]; (d) [(PPh3)2N]4[Au12Ag32(SPhF)30] heated in air at 80 ℃ for different time. | |
Similar to [Ag44(SR)30]4- clusters,the stability of [Au12Ag32(SR)30]4- clusters depended on the surface ligands and counter cations. The change of surface ligands from -SPhF2 to -SPhCF3 and -SPhF reduced the stability of the clusters. At 80 ℃ in air,(PPh4)4[Au12Ag32(SPhCF3)30] was stable for 4 days (Fig. 4b), (PPh4)4[Au12Ag32(SPhF)30] started to degrade beyond 24 h (Fig. 4c). Similar to [Ag44(SR)30]4- clusters,the DMF solutions of the (PPh4)4[Au12Ag32(SR)30] eventually turned brown-red to yellow and displayed only a sharp peak at 460 nm when the heating time at 80 ℃ was long enough. The nanoclusters were aggregated to form larger nanoparticles (Fig. S9 in Supporting information). Also, replacing the counter cations PPh4 + with more bulky [(PPh3)2N]+ cations significantly reduced the stability of the [Au12Ag32(SR)30]4- clusters. As shown in Fig. 4d,[(PPh3)2N]4[Au12Ag32(SPhF2)30] was stable in DMF for only 40 h at 80 ℃ in air,much shorter than that (>8 days) for (PPh4)4[Au12Ag32(SPhF2)30].
4. ConclusionIn summary,series of [M12Ag32(SR)30]4- (M = Ag,Au) clusters with -SPhF2,-SPhCF3 or -SPhF as surface ligands,and PPh4 + or [(PPh3)2N]+ as counter cations have been prepared and used as a nice system to demonstrate how subtle structural variation of surface ligands and counter cations were important in determining the stability of anionic thiolated noble metal nanoclusters. While 3,4-difluorobenzenethiol was demonstrated as a better surface ligand than 4-(trifluoromethyl)benzenethiol or 4-fluorobenzenethiol to stabilize [M12Ag32(SR)30]4-,the use of more bulky [(PPh3)2N]+ as the counter cations was found to be more deleterious to the overall stability of [M12Ag32(SR)30]4- clusters than PPh4 +. With the same surface ligands and counter cations, [Au12Ag32(SR)30]4- were much more stable than [Ag44(SR)30]4-. Although the electronic shell closing helped to explain the stability of many noble metal nanoclusters,the superatom theory could not provide insights on the origins of the stability differences among the same clusters with subtle structural variations. More efforts should be made to understand why a small structural change could result in a significant change in their stability.
AcknowledgmentsWe thank the MOST of China (No. 2011CB932403) and the NSFC of China (Nos. 21131005,21390390,21333008) for financial support.
Appendix A. Supplementary dataCrystallographic data as .cif files for the structures reported in this paper have been deposited with the Cambridge Crystallographic Data Center. CCDC numbers are 1002193,1002194, 1002195 and 1002196 for [(PPh3)2N]4[Au12Ag32(SC6H3F2)30], [(PPh3)2N]4 [Au12Ag32(SC6H4CF3)30],[(PPh3)2N]4[Ag44(SC6H4CF3)30] and [(PPh3)2N]4[Ag44(SC6H3F2)30],respectively. Copies of the data canbe obtained free of charge fromCCDC,12UnionRoad,Cambridge CB2 1EZ,UK. Syntheses,crystallographic data and the crystal structures of [(PPh3)2N]4[Au12Ag32(SR)30],UV-vis spectra of [Au12Ag32(SR)30]4 - clusters with different surface thiolates,and TEM images of (PPh4)4[Au12Ag32(SPhF2)30] clusters under different treatment conditions are listed in Supporting informaiton. Supplementary data associatedwith this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.05.027.
| [1] | M.C. Daniel, D. Astruc, Gold nanoparticles: assembly, supramolecular chemistry, quantuμ-size-related properties, and applications toward biology, catalysis, and nanotechnology, Chem. Rev. 104 (2003) 293-346. |
| [2] | C.M. Niemeyer, Nanoparticles, proteins, and nucleic acids: biotechnology meets materials science, Angew. Chem. Int. Ed. 40 (2001) 4128-4158. |
| [3] | T. Tsukuda, Toward an atomic-level understanding of size-specific properties of protected and stabilized gold clusters, Bull. Chem. Soc. Jpn. 85 (2012) 151-168. |
| [4] | N.F. Zheng, G.D. Stucky, A general synthetic strategy for oxide-supported metal nanoparticle catalysts, J. Am. Chem. Soc. 128 (2006) 14278-14280. |
| [5] | A.C. Templeton, W.P. Wuelfing, R.W. Murray, Monolayer-protected cluster molecules, Acc. Chem. Res. 33 (1999) 27-36. |
| [6] | D.A. Giljohann, D.S. Seferos, W.L. Daniel, et al., Gold nanoparticles for biology and medicine, Angew. Chem. Int. Ed. 49 (2010) 3280-3294. |
| [7] | R.C. Jin, Quantum sized, thiolate-protected gold nanoclusters, Nanoscale 2 (2010) 343-362. |
| [8] | P.D. Jadzinsky, G. Calero, C.J. Ackerson, D.A. Bushnell, R.D. Kornberg, Structure of a thiol monolayer-protected gold nanoparticle at 1.1 a resolution, Science 318 (2007) 430-433. |
| [9] | M.Z. Zhu, C.M. Aikens, F.J. Hollander, G.C. Schatz, R. Jin, Correlating the crystal structure of a thiol-protected Au25 cluster and optical properties, J. Am. Chem. Soc. 130 (2008) 5883-5885. |
| [10] | M.W. Heaven, A. Dass, P.S. White, K.M. Holt, R.W. Murray, Crystal structure of the gold nanoparticle [N(C8H17)4][Au25(SCH2CH2Ph)18], J. Am. Chem. Soc. 130 (2008) 3754-3755. |
| [11] | H.Y. Yang, Y. Wang, H.Q. Huang, et al., All-thiol-stabilized Ag44 and Au12Ag32 nanoparticles with single-crystal structures, Nat. Commun. 4 (2013), Article 2422. |
| [12] | H.F. Qian, C. Liu, R.C. Jin, Controlled growth of molecularly pure Au25(SR)18 and Au38(SR)24 nanoclusters from the same polydispersed crude product, Sci. China Chem. 55 (2012) 2359-2365. |
| [13] | M. Walter, J. Akola, O. Lopez-Acevedo, et al., A unified view of ligand-protected gold clusters as superatom complexes, Proc. Natl. Acad. Sci. U. S. A. 105 (2008) 9157-9162. |
| [14] | Y. Negishi, K. Munakata, W. Ohgake, K. Nobusada, Effect of copper doping on electronic structure, geometric structure, and stability of thiolate-protected Au25 nanoclusters, J. Phys. Chem. Lett. 3 (2012) 2209-2214. |
| [15] | M.A. Tofanelli, C.J. Ackerson, Superatom electron configuration predicts thermal stability of Au25(SR)18 nanoclusters, J. Am. Chem. Soc. 134 (2012) 16937-16940. |
| [16] | X. Chen, M. Strange, H. Häkkinen, Nonmagnetic and magnetic thiolate-protected Au25 superatoms on Cu(111), Ag(111), and Au(111) surfaces, Phys. Rev. B 85 (2012) 085422. |
| [17] | H. Häkkinen, Atomic and electronic structure of gold clusters: understanding flakes, cages and superatoms from simple concepts, Chem. Soc. Rev. 37 (2008) 1847-1859. |
| [18] | S. Knoppe, S. Malola, L. Lehtovaara, T. Bürgi, H. Häkkinen, Electronic structure and optical properties of the thiolate-protected Au28(SMe)20 cluster, J. Phys. Chem. A 117 (2013) 10526-10533. |
| [19] | E.B. Guidez, V. Makinen, H. Häkkinen, C.M. Aikens, Effects of silver doping on the geometric and electronic structure and optical absorption spectra of the Au25-nAgn(SH)18- (n=1,2, 4, 6, 8, 10, 12) bimetallic nanoclusters, J. Phys. Chem. C 116 (2012) 20617-20624. |
| [20] | R. Guo, R.W. Murray, Substituent effects on redox potentials and optical gap energies of molecule-like Au38(SPhX)24 nanoparticles, J. Am. Chem. Soc. 127 (2005) 12140-12143. |
| [21] | F. Chen, R.L. Johnston, Energetic, electronic, and thermal effects on structural properties of Ag-Au nanoalloys, ACS Nano 2 (2007) 165-175. |
| [22] | W. Kurashige, M. Yamaguchi, K. Nobusada, Y. Negishi, Ligand-induced stability of gold nanoclusters: thiolate versus selenolate, J. Phys. Chem. Lett. 3 (2012) 2649-2652. |
| [23] | J. Jung, S. Kang, Y.K. Han, Ligand effects on the stability of thiol-stabilized gold nanoclusters: Au25(SR)18-, Au38(SR)24, and Au102(SR)44, Nanoscale 4 (2012) 4206-4210. |
| [24] | Y. Negishi, W. Kurashige, Y. Niihori, T. Iwasa, K. Nobusada, Isolation, structure, and stability of a dodecanethiolate-protected Pd1Au24 cluster, Phys. Chem. Chem. Phys. 12 (2010) 6219-6225. |
| [25] | D.E. Jiang, S. Dai, From superatomic Au25(SR)18- to superatomic M@Au24(SR)18q core-shell clusters, Inorg. Chem. 48 (2009) 2720-2722. |
| [26] | S.L. Christensen, M.A. MacDonald, A. Chatt, et al., Local structure, and electronic properties of Au24Pt(SR)18 nanoclusters, J. Phys. Chem. C 116 (2012) 26932-26937. |




