As an important class of organic-inorganic hybrid materials, metal phosphonates have attracted much attention in the past decades due to their rich structural chemistry,high thermal stability and potential applications in catalysis [1],ion-exchange [2, 3],proton conductivity [4, 5] and magnetism etc. [6, 7]. Cobalt phosphonates are of particular interest especially in magnetism because of the large single ion magnetic anisotropy [8]. A number of cobalt phosphonates have been reported to date,some of which display interesting properties such as metamagnetism [9, 10],weak ferromagnetism [11, 12],ferrimagnetism [13] and tunable magnetic behaviors [14]. The rigid aromatic carboxylatophosphonate ligands such as 2-/3-/4-carboxylatophenylphosphonate [15, 16, 17], phosphonoisophthalic acid [18, 19] and carboxylatopyridylphosphonate [20, 21] are efficient in building cobalt phosphonates with versatile architectures. More recently,two cobalt phosphonates Co2(pbtcH)(2,20-bpy)2(H2O) and Co2(pbtcH)(phen)2(H2O) based on 5-phosphonatophenyl-1,2,4-tricarboxylic acid (pbtcH5,Scheme 1a) have been isolated,which experience single-crystal to singlecrystal (SC-SC) structural transformation upon dehydration/ rehydration in a reversible manner [22]. In this paper,we report two new related compounds using the same ligand as the starting material,namely,M2(pbtcH)(phen)2(H2O)2 [M(II) = Co (1),Ni (2)]. Compounds 1 and 2 show double-chain structures,which are completely different from that of Co2(pbtcH)(phen)2(H2O). The IR, thermal stability and magnetic properties are investigated.
2. ExperimentalAll reagents were commercially available and were used as received. The ligand 5-phosphonatophenyl-1,2,4-tricarboxylic acid (pbtcH5) was synthesized according to the published procedure [22]. Elemental analysis was performed with an Elementar Vario MICRO elemental analyzer. The room temperature infrared spectra were performed on a Bruker Vector 27 instrument. The powder χ-ray diffraction (PXRD) data were collected on a Bruker D8 advance χ-ray diffractometer with Cu- Ka radiation (λ = 1.54056Å ). Thermogravimetric analyses were carried out on a Mettler-Toledo TGA/DSC1 instrument in the range of 25-800 ℃ under nitrogen flow at a rate of 10 ℃ min-1.
The GAUSSIAN 03 program package was employed to carry out DFT calculations at the Becke’s three-parameter functional and Lee-Yang-Parr functional (B3LYP) [23, 24] levels of calculation and the 6-31G(d,p) and LANL2DZ basis sets were used for the frequency calculations of the complex. The coordinates obtained from the single crystal χ-ray diffraction data were used directly.
Synthesis of Co2(pbtcH)(phen)2(H2O)2 (1): A mixture of CoCl2·6H2O (0.15 mmol,0.0248 g),pbtcH5 (0.05 mmol,0.0145 g), phen (0.15 mmol,0.0100 g) and H2O (4 mL),adjusted to pH ≈ 2.7 with 0.1 mol L-1 piperazidine,was placed in a 25 mL teflon-lined stainless steel vessel. The mixture was sealed and heated at 180 ℃ for 24 h,then the reaction system was cooled to room temperature. Mauve column crystals of 1 were obtained as a pure phase in a yield of 20% (based on Co). Element analysis calcd. (%) for C33H23Co2N4O11P: C,49.52; H,2.90; N,7.00; found: C,49.74; H,2.39; N,7.05. IR (KBr,cm-1): 3448(w), 3072(w),1625(w),1604(w),1581(s),1544(s),1514(s), 1429(vs),1381(s),1321(m),1182(vs),1079(s),979(w), 927(w),893(s),856(vs),785(m),729(vs),683(w),640(w), 607(w),554(w),530(w).
Synthesis of Ni2(pbtcH)(phen)2(H2O)2 (2): Compound 2 was synthesized following the same procedure as that for compound 1 except that CoCl2·6H2O was replaced by NiCl2·6H2O. Element analysis calcd. (%) for C33H23Ni2N4O11P: C,49.55; H,2.90; N,7.00; found: C,49.71; H,2.43; N,7.03. IR (KBr,cm-1): 3449(w),3069(w), 1627(w),1604(w),1585(m),1573(m),1539(s),1514(s),1429(vs), 1381(s),1321(vs),1180(vs),1157(s),1136(m),1082(s),977(w), 927(w),891(s),852(vs),785(m),729(vs),682(w),644(w),609(w), 553(w),530(w).
Crystal data for 1: C33H23Co2N4O11P,Mr = 800.38,triclinic, space group P-1,a = 7.397(1),β = 9.877(2),c = 10.604(1) Å , α = 84.067(3)8,β = 83.328(2)8,γ = 74.486(3)8,V = 739.3(2) Å 3, Z = 1,GOF = 1.055. For 2: C33H23Ni2N4O11P,Mr = 799.94,triclinic, space group P-1,a = 7.351(1),β = 9.863(2),c = 10.597(2) Å , α = 84.254(3)8,β = 83.740(3)8,γ = 74.579(3)8,V = 734.2(2) Å 3, Z = 1,GOF = 0.919. The data collection was carried on a Bruker SMART APEX CCD diffractometer equipped with graphite-monochromatized Mo Ka (λ = 0.71073Å ). The structures were solved by the direct methods and refined on F2 by full-matrix least squares using SHELXTL [25],and converging at R1 = 0.0623,wR2 = 0.1403 for 1 and R1 = 0.0630,wR2 = 0.1420 for 2,respectively. CCDC 991621-991622 contain the crystallographic data of the two compounds.
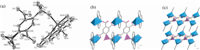
3. Results and discussionCompounds 1 and 2 are isostructural,crystallizing in the triclinic space group P-1. Therefore the structure of compound 1 is described as a representative. As shown in Fig. 1a,the asymmetric unit of 1 consists of one Co,0.5 pbtcH4-,one phen and one aqua molecule. The Co atom has a distorted octahedral coordination environment,surrounded by two nitrogen (N1,N2) atoms from phen,two carboxylate oxygen atoms (O6,O7),one disordered oxygen (O1 or O4) atom from pbtcH4-,and one aqua ligand (O1w). The Co-O and Co-N bond lengths are in the range of 1.957(16)- 2.213(4) Å and 2.100(4)-2.124(5) Å ,respectively.
The pbtcH4- acts as a hexadentate ligand,chelating and bridging four Co atoms through six of its nine oxygen donors. A crystallographic inversion center is located at the center of the benzene ring. Hence the phosphonate group and one carboxylate group (at the para-position) of the pbtcH4- are disordered over two positions,with the occupancies of 50% each. One phosphonate oxygen atom (O2) is protonated. Each pbtcH4- chelates to the Co through the carboxylate group (O6,O7),and the disordered phosphonate/carboxylate oxygen (O1/O4),thus forming an infinite double chain running along the a-axis (Fig. 1b). Neighboring chains are connected by extensive hydrogen bonding interactions among the coordinated water and phosphonate/carboxylate oxygen atoms,resulting in a layer in the ab plane (Fig. S5 in Supporting information). The coordinated water molecule (O1w),pendent carboxylate oxygen and phosphonate oxygen are involved in the intra-chain hydrogen bonds. The shortest O. . .O distances within the layer between the chains are 2.82(2) Å for O1w. . .O5i and 2.63(1)Å for O1w. . .O3i. The hydrogen bonds involved in the interlayer space are also found [O1w. . .O3ii: 2.63(1) Å ; O1w. . .O5ii: 3.01(2)Å] (symmetry codes: i: 1-x,1-y,-z; ii: -1 + x,y,z). The layers are stacked via p-p interactions between the two rings of phen from adjacent layers,with a centroid-centroid distance of 3.57Å and an inter-plane angle of 1.98 [26]. Therefore,a threedimensional supramolecular structure is constructed (Fig. 1c).
The structure of compound 2 is identical to that of 1. The Ni-O and Ni-N bond lengths are in the range of 1.953(16)-2.160(4) Å and 2.049(4)-2.069(5) Å ,respectively. Between the chains,the shortest O. . .O distances within the layer are 2.84(2) Å for O1w. . .O5iii,2.70(2) Å for O1w. . .O3iii and 3.11(2) Å for O2. . .O5iii. iii. (symmetry code: iii: 2-x,-y,-z). The hydrogen bonds also exist between the layers [O1w. . .O3ii: 2.66(2) Å]. Interactions of p-p staking between the two rings of phen from adjacent layers are present with a centroid-centroid distance of 3.58Å and an interplane angle of 2.48.
The double chain structures of 1 and 2 are significantly different from those of Co2(pbtcH)(2,20-bpy)2(H2O) and Co2(pbtcH)(phen)2(H2O) [22]. The latter compounds were obtained at a lower temperature (140 ℃) and show layered structures in which the tetramers of Co4 are cross-linked by pbtcH4- into a layer. Obviously,slight changes in the experimental conditions could influence significantly the final products. Similar structures have not been found in the other cobalt or nickel phenylphosphonates containing single or double carboxylate groups,attributed to the different coordination modes of pbtcH4- compared with the other related phosphonate ligands [15, 16, 17, 18, 19, 20, 21].
Thermal analyses reveal that compounds 1 and 2 are stable up to 190 ℃ and 280 ℃,respectively (Fig. S6 in Supporting information). For 1,the weight loss of 4.5% in the temperature range 190- 275 ℃ is close to the calculated value for the removal of two water molecules (calcd. 4.5%). The weight loss above 275 ℃ is attributed to the decomposition of the organic groups and the collapse of the structure. For compound 2,the first-step weight loss in the temperature range 280-340 ℃ is 5.7%,larger than the value expected for the release of two water molecules (calcd. 4.5%). This is followed immediately by the next-step weight loss,corresponding to the decomposition of the organic groups and the collapse of the structure.
The infrared spectra of 1 and 2 reveal that the stretching vibration peaks for the phosphonate groups,which normally appear in the range of 1140-970 cm-1,cannot be clearly identified. In order to assign the vibration peaks,DFT calculation on the structural unit of 1 was carried out using the GAUSSIAN 03 program package at the level of B3LYP. The calculated IR spectra correlate fairly well with the experimental spectra in the relative locations of the vibration peaks (Fig. S1 in Supporting information). Accordingly,the bands located at 3452 and 3065 cm-1 can be assigned to the stretching vibration of O-H in the aqua ligand and the phosphonate group,respectively. The peaks appearing at 1581 cm-1 and 1382 cm-1 are assigned to the asymmetric and symmetric stretching vibration of the carboxylate group (mode A), and those appearing at 1500 cm-1 and 1426 cm-1 are assigned to the asymmetric and symmetric vibration of n(COO) (mode B) (Scheme 1b). The vibration bands related to phen are located at 1617 cm-1,1608-1526 cm-1,1479-1417 cm-1,1375-1344 cm-1 and 1296-1289 cm-1. Those related to the phosphonate group are very weak,in agreement with the experimental results.

|
Download:
|
| Scheme 1.(a) Molecular structure of pbtcH5. (b) Coordination modes of pbtcH5. | |
|
Download:
|
| Fig. 1. Building unit of structure 1 (50% thermal ellipsoids),all hydrogen atoms are omitted for clarity. (b) One chain of structure 1 running along the a-axis. (c) Structure 1 packed along the b-axis. | |
The temperature dependent molar magnetic susceptibility of both compounds is measured in the temperature range of 1.8- 300 K at 2 kOe. The observed room temperature xMT per Co or Ni is 3.10 cm3 K mol-1 and 1.30 cm3 K mol-1 for compounds 1 and 2, respectively (Fig. 2). The former is much larger than the expected spin-only value of 1.88 cm3 K mol-1 for spin S = 3/2 with γ = 2.0, attributed to the orbital contribution of the octahedral cobalt ion. Above 50 K,the susceptibility data obey the Curie-Weiss law in both cases. The Curie and Weiss constants are 3.21 cm3 K mol-1 and -9.15 K for 1,and 1.32 cm3 K mol-1 and -6.28 K for 2, respectively. Upon cooling from room temperature,the xMT value decreases continuously until it reaches a minimum of 1.62 cm3 K mol-1 for 1 and 0.35 cm3 K mol-1 for 2 at 1.8 K. The magnetization curves were measured at 1.8 K. The value at 70 kOe is 2.57 Nb per Co for 1,which is slightly higher than the saturation value of 2.3 Nb expected for one Co(II) ion. For 2,the magnetization is not saturated at 70 kOe with a value of 1.73 Nb per Ni (Figs. S7, S8 in Supporting information).
Considering that in compounds 1 and 2 the metal ions are linked by the pbtcH4- ligand,the exchange coupling through this pathway should be very weak. Thus the magnetic behaviors may be well explained by a model for single metal ion. For compound 1, the magnetic susceptibility data can be fit by Eqs. (1) and (2) for single Co(II) ion where x = l/kT,l is the spin-orbit coupling constant,A is a crystal field parameter,and u accounts for exchange couplings between the Co(II) ions [27]. The best fit for the data above 50 K leads to parameters l = -152 cm-1,A = 1.05,and u = 9.5 K (Fig. 2a). The positive u value suggests the presence of weak ferromagnetic interactions between the Co(II) centers.


For compound 2,the negative u value is indicative of a weak antiferromagnetic interactions between the Ni(II) ions. The magnetic susceptibility data can be simulated using a simple mononuclear model for spin S = 1 considering the zero-field splitting parameter (D) and the inter-molecular interaction (zJ) [28]:


The best fit for the data in the whole temperature range results in parameters D = 0.54 cm-1 and zJ = -2.3 cm-1 (g is fixed at 2.18) (Fig. 2b).
4. ConclusionTwo new metal phosphonates M2(pbtcH)(phen)2(H2O)2 [M(II) = Co (1),Ni (2)] based on 5-phosphonatophenyl-1,2,4-tricarboxylate are reported. Both show chain structures in which the metal ions are connected by the phosphonate ligands. The co-ligands of phen are placed on the two sides of the chain. It is reasonable to hypothesize that the substitution of phen by a neutral bridging ligand may expand the dimension of the structure. Investigations are in progress to construct mixed-ligated compounds based on the same phosphonate ligand but with open-framework structures.
AcknowledgmentsFinancial supports by the National Basic Research Program of China (No. 2013CB922102) and the NSF of Jiangsu Province of China (No. BK2009009) are acknowledged.
Appendix A. Supplementary dataSupplementary material related to this article can be found,in the online version,at http://dx.doi.org/10.1016/j.cclet.2014.05.005
| [1] | P.O. Adelani, T.E. Albrecht-Schmitt, Differential ion exchange in elliptical uranyl diphosphonate nanotubules, Angew. Chem. Int. Ed. 49 (2010) 8909-8911. |
| [2] | M. Plabst, L.B. McCusker, T. Bein, Exceptional ion-exchange selectivity in a flexible open framework lanthanum(III) tetrakisphosphonate, J. Am. Chem. Soc. 131 (2009) 18112-18118. |
| [3] | J.M. Taylor, R.K. Mah, I.L. Moudrakovski, et al., Facile proton conduction via ordered water molecules in a phosphonate metal-organic framework, J. Am. Chem. Soc. 132 (2010) 14055-14057. |
| [4] | X. Liang, F. Zhang, W. Feng, et al., From metal-organic framework (MOF) to MOFpolymer composite membrane: enhancement of low-humidity proton conductivity, Chem. Sci. 4 (2013) 983-992. |
| [5] | Y.Z. Zheng, M. Evangelisti, F. Tuna, R.E.P. Winpenny, Co-Ln Mixed-metal phosphonate grids and cages as molecular magnetic refrigerants, J. Am. Chem. Soc. 134 (2011) 1057-1065. |
| [6] | Y. Zhang, X.B. Han, Z.M. Zhang, et al., A {Ni7} cluster-containing sandwich-type phosphotungstate functionalized by organic bisphosphonate ligands and its two-dimensional supramolecular structure, Chin. Chem. Lett. 24 (2013) 581-584. |
| [7] | L.M. Zheng, Y. Duan, Structural and magnetic studies of cobalt phosphonates, in: A. Clearfield, K. Demadis (Eds.), Metal Phosphonate Chemistry: From Synthesis to Applications, the Royal Society of Chemistry (2012) 235-278. |
| [8] | T.H. Yang, Y. Liao, L.M. Zheng, et al., Tuning the field-induced magnetic transition in a layered cobalt phosphonate by reversible dehydration-hydration process, Chem. Commun. (2009) 3023-3025. |
| [9] | Z.S. Cai, S.S. Bao, L.M. Zheng, Layered cobalt phosphonate with metamagnetism, Acta Chim. Sinica 71 (2013) 555-559. |
| [10] | B.P. Yang, A.V. Prosvirin, Y.Q. Guo, J.G. Mao, Co[HO2C(CH2)3NH(CH2PO3H)2]2: a new canted antiferromagnet, Inorg. Chem. 47 (2008) 1453-1459. |
| [11] | J. Huang, S.S. Bao, L.S. Ling, et al., A racemic polar cobalt phosphonate with weak ferromagnetism, Chem. Eur. J. 18 (2012) 10839-10842. |
| [12] | L.R. Guo, S.S. Bao, B. Liu, et al., Enhanced magnetic hardness in a nanoscale metalorganic hybrid ferrimagnet, Chem. Eur. J. 18 (2012) 9534-9542. |
| [13] | S.S. Bao, Y. Liao, Y.H. Su, et al., Tuning the spin state of cobalt in a Co-La heterometallic complex through controllable coordination sphere of La, Angew. Chem. Int. Ed. 50 (2011) 5504-5508. |
| [14] | P.F. Wang, D.K. Cao, S.S. Bao, et al., Co3(2-OOCC6H4PO3)2(H2O)3 H2O: a layered metal phosphonate showing reversible dehydration-rehydration behavior and ferrimagnetism, Dalton Trans. 40 (2011) 1307-1312. |
| [15] | J.T. Li, T.D. Keene, D.K. Cao, S. Decurtins, L.M. Zheng, [M(OOCC6H4PO3H)(H2O)] (M(II)=Mn, Co, Ni): layered metal phosphonates showing variable magnetic behavior, CrystEngComm 11 (2009) 1255-1260. |
| [16] | J.M. Rueff, V. Caignaert, S. Chausson, et al., meta-Phosphonobenzoic acid: a rigid heterobifunctional precursor for the design of hybrid materials, Eur. J. Inorg. Chem. (2008) 4117-4125. |
| [17] | P.F. Wang, Y. Duan, T.W. Wang, Y.Z. Li, L.M. Zheng, Three-dimensional metal phosphonodicarboxylates with GIS-zeolite topology: syntheses, structures and magnetic studies, Dalton Trans. 39 (2010) 10631-10636. |
| [18] | H.J. Jin, P.F. Wang, S.S. Bao, L.M. Zheng, C. Yao, Layered manganese 4-phosphonoisophthalates (4-piH4) embeding Mn-O chains with metamagnetism in Mn3(4-piH)2(H2O)3•H2O, Sci. China Chem. 55 (2012) 1047-1054. |
| [19] | P.F. Wang, Y. Duan, J.M. Clemente-Juan, et al., Magnetization relaxation in a threedimensional ligated cobalt phosphonate containing ferrimagnetic chains, Chem. Eur. J. 17 (2011) 3579-3583. |
| [20] | P.F. Wang, Y. Duan, L.M. Zheng, One-dimensional metal phosphonates based on 6-phosphononicotinic acid: a structural and magnetic study, Sci. China Chem. 53 (2010) 2112-2117. |
| [21] | T. Zheng, J.M. Clemente-Juan, J. Ma, et al., Breathing effect in a cobalt phosphonate upon dehydration/rehydration: a single-crystal-to-single-crystal study, Chem. Eur. J. 19 (2013) 16394-16402. |
| [22] | A.D. Becke, Density-functional exchange-energy approximation with correct asymptotic behavior, Phys. Rev. A 38 (1988) 3098-3100. |
| [23] | C. Lee, W. Yang, R.G. Parr, Development of the Colle-Salvetti correlation-energy formula into a functional of the electron-density, Phys. Rev. B 37 (1988) 785-789. |
| [24] | SHELXTL (version 5.0), Reference Manual, Siemens Industrial Automation, Analytical Instruments, Madison, WI, 1995. |
| [25] | C. Janiak, A critical account on π-π stacking in metal complexes with aromatic nitrogen-containing ligands, J. Chem. Soc. Dalton Trans. (2000) 3885-3896. |
| [26] | F.E. Mabbs, D.J. Machin, Magnetism and Transition Metal Complexes, Chapman and Hall, London, 1973, p. 99. |
| [27] | O. Kahn, Molecular Magnetism, VCH Publishers, Weinheim, 1993. |




