b School of Chemical and Biological Engineering, Lanzhou Jiaotong University, Lanzhou 730070, China
Metal-organic frameworks (MOFs) are assembled by linking inorganic and organic building bl℃ks through coordination interactions [1],and the development of MOFs in recent years makes it possible that crystalline MOF architectures can be assembledfromwell-definedmolecularcomponents[2].However, the knowledge about how to precisely control the structure of MOFs on the molecular level is still in its infancy [3]. Therefore, rational prediction,design,and synthesis of MOFs are currently of great interest in the field of coordination chemistry and crystal engineering because of their fascinating structures [4],intriguing variety of topologies [3b, 5],as well as their interesting properties such as gas absorption,catalysis,ion exchange,non-linear optics, and magnetism [6]. Accordingly,the synthesized MOFs with desired structures and properties should meet optimal require- ments,for example phase purity,suitable pore size and volume, retention of framework,and porosity upon removal of guest molecules. In practice,it is difficult to fully satisfy such requirements,because the assembly of MOFs can be influenced by not only the organic ligands and metal centers [7],but also the reaction temperature,pH value,molar ratio of reactants, solvent system,and counter ions,which makes the controllable preparation of the target MOFs still a great challenge [8]. Among the above mentioned reaction conditions,the reaction tempera- ture is one of the key parameters in the synthesis of MOFs based on the following reasons: firstly,the reaction temperature affects the solubility of the organic ligand; secondly,flexible organic ligands inherently have the potential to adopt different conformations underdifferenttemperatures[9];thirdly,thereactiontemperature may play a crucial role in tuning the coordination mode of organic ligands,especially the carboxylate ligand [10, 11]; and finally,the reaction temperature affects directly the reaction energy barrier in reaction thermodynamics and the reaction rate in the reaction kinetics. Consequently,the reaction temperature can be used as a structure-directing factor and multidimensional architectures can be purposefully obtained. This review aims to illustrate the influence of reaction temperature on the assembly of MOFs and to provide some primary information for the design and assembly of the desired MOFs.
2. Reaction temperature effect on the assembly of MOFsHydro/solvothermal reactions are well used in the synthesis of MOFs,and in such reaction systems,it is evident that high temperature will produce high reaction pressure in the sealed system,and accordingly will affect the assembly and final architecture of MOFs. It has been reported that increasing the hydro/solvothermal reaction temperature tends to increase the coordinationnumber of the central metalion and the dimensionality of the MOFs,and at the same time to reduce the coordinated solvent molecules [12].
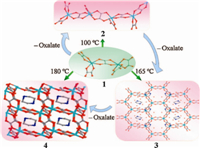
The reported works have shown that the dimensionality of MOFs can be regulated by adjusting the reaction temperature [13- 16]. A typical example is that entirely different Co(II)-succinates MOFs were obtained by using the same starting reaction mixture, but different reaction temperature,and the results show that the dimensionality and density of the MOFs increased when the reaction temperature was raised [14]. Five Co(II)-succinates MOFs were isolated by reaction of cobalt hydroxide,succinic acid and water in approximately 1:1:28 ratio at 60,100,150,190 and 250 ℃. The dimensionality of the resulting Co(II)-MOFs changes from one-dimensional (1D) chains obtained at 60 ℃ and 100 ℃,to two-dimensional (2D) networks at 150 ℃,and to three-dimen- sional (3D) frameworks at 190 ℃ and 250 ℃. Moreover,the density of the MOFs increased and the coordinated water molecules decreased with raising the reaction temperature. Dan and Rao reported a progressive increase in dimensionality using a zero-dimensional (0D) dimeric zinc(II) oxalate complex, (C4N2H12)3[Zn2(C2O4)5]·8H2O (1) (C4N2H12= piperazine dication) (Fig. 1) [15]. MOFs (C4N2H12)2[Zn2(C2O4)4]·3H2O (2), (C4N2H12)3[Zn4(C2O4)7]·4H2O (3),and (C4N2H12)[Zn2(C2O4)3] (4) were successfully isolated on heating 1 in the presence of piperazine (PIP) at 100,165,and 180 ℃,respectively. Interestingly, 2 has a 1D infinite chain structure,3 is a pseudo-2D with honeycomb apertures while 3 is a 3D architecture. It is considered that the 3D structure emerges from the building-up of the lower- dimensional structures with eliminating the oxalate moiety as illustrated in Fig. 1. This study further demonstrates that with increasing the reaction temperature,the overall dimensionality of the MOFs increases. Similar phenomena have been observed in our and other reported MOFs [16].
|
Download:
|
| Fig. 1. Assembly of MOFs 2-4 controlled by temperature,and the formation of 1D chain (2),pseudo-2D (3),and 3D (4) structures from 0D (1). | |
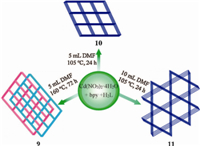
Frameworks [Zn(tib)2(H2O)](ClO2)2(5) and [Zn4(tib)2(PB- DA)3(OH)2]·4H2O (6) were obtained by using mixed organic ligands of 1,3,5-tris(1-imidazolyl)benzene (tib) and p-benzenedi- carboxylic acid (H2PBDA) under the same conditions except for the different reaction temperatures of 140 ℃ for 5 and 180 ℃ for 6,and the case of [Zn2(tib)(PBDA)Cl2]·2H2O (7) and [Zn4(tib)2(PBDA)3Cl2] (8) has the same situation (Fig. 2) [17]. MOFs 5 and 7 obtained at the lower temperature of 140 ℃ are 2D networks with different topologies,while 6 and 8 synthesized at the higher temperature of 180 ℃ are 2-fold interpenetrated 3D frameworks (Fig. 2). The structural difference between 5 and 6 as well as 7 and 8 implies the great influence of the hydrothermal reaction temperature on the structure of MOFs.When the reaction temperature was raised from 140 ℃ to 180 ℃,the architectures varied from non-interpene- trated 2D networks of 5,7 to interpenetrated 3D frameworks of 6,8.It implies that a high reaction temperature may benefit to form multidimensional frameworks with large voids,and hence may tend to form interpenetrated structures [18]. Xu and his coworkers reported for the first time the non-,micro- and meso-porous MOF isomers achieved by interpenetration control via lowering reaction temperature and decreasing reactant concentration [19]. [Cd(L)(bpy)] (9),[Cd(L)(bpy)]·4H2O·2.5DMF (10) and [Cd(L)(bpy)]·4.5H2O·3DMF (11) were synthesized by solvothermal reactions of Cd(NO3)2·4H2O,4,4 0 -bipyridine (bpy) and 2-amino-1,4-benzenedicarboxylic acid (H2L) in DMF (N,N- dimethylformamide). As shown in Fig. 3,non-porous 9 obtained in 5 mL DMF at 160 ℃ for 72 h possesses a 2-fold interpenetrated 3D framework,micro-porous 10 isolated in 5 mL DMF at 105 ℃ for 24 h has a similar framework structure with the single net of 9,namely,the interpenetration is suppressed by lowering the reaction temperature,and the meso-porous 11 prepared in 10 mL DMF at 105 ℃ for 24 h is a 3D open framework with large open channels realized by decreasing the concentration of the reactants. Further examples were found in the reported Co(II)- succinate and Mn(II)-BPTCA 4· [BPTCA 4· = 4,4 0 -bipyridine- 2,2 0 ,6,6 0 -tetracarboxylate] MOFs [20].

|
Download:
|
| Fig. 2. Schematic diagram of the synthetic conditions and topology for MOFs 5-8. | |
|
Download:
|
| Fig. 3. Synthetic conditions and topological representation of MOFs 9-11. | |
In addition,the organic ligands can adopt different conformation and coordination mode under different reaction temperature as reported by Zhang and Bu’s groups [21]. For example,assembly of 4-(4-carboxyphenylamino)-3,5-dinitrobenzoic acid (H2cpdba) and 2,20-bipyridine (2,20-bpy) with Mn(II) salt at different reaction temperatures gave rise to three different MOFs [21a]. {[Mn(cpdba)(2,20-bpy)]}n (12) obtained at 140 ℃ displays a (4,4) 2D network,while {[MN2(cpdba)2(2,20-bpy)(H2O)2]·3H2O}n (13) isolated at 150 ℃ possesses a 3D framework with unique Mn3(cpdba)6(2,20-bpy)2 building bl℃ks,and with further increasing reaction temperature to 170 ℃,3D framework {[Mn4(cpdba)4(2,20-bpy)3(H2O)2]}n (14) is formed with decreased coordinated and lattice water molecules compared with those of 13. It is noteworthy that the coordination modes of the cpdba2- ligand are different in MOFs 12-14 as schematically shown in Fig. 4. When the dimensionality of the frameworks is increased from 2D (12) to 3D (13 and 14),one carboxylate group of the cpdba2- ligands in 13 and 14 shows an additional μ2-η2:η1- coordination mode (Fig. 4c). The results suggested that the carboxylate groups tend toward an increase in the coordination number of metal ions and the formation of the M-O-M linkage mode with increasing reaction temperature.

|
Download:
|
| Fig. 4. Coordination modes of cpdba2- ligand in 12 (a) and 13,14 (b and c). | |
There are also examples of MOFs without dimensionality change of the architectures under different reaction temperature, however,their structures differ greatly. For example,the reaction of Co(NO3)2·6H2O with 5-bromoisophthalic acid (H2BIPA) and 1,3,5-tris(imidazol-1-ylmethyl)-2,4,6-trimethylbenzene (titmb) in aqueous solution at 120 ℃ gave MOF [Co(BIPA)(titmb)]·H2O (15) in which the Co(II) has a square-pyramidal coordination geometry (Fig. 5a),while the same reaction carried out at 180 ℃,complex [Co3(BIPA)3(titmb)2]·0.73H2O (16) with the Co(II) adopting distorted tetrahedral coordination geometry (Fig. 5b and c) was obtained [22]. MOFs 15 and 16 are polymorphs of 3D frameworks, in which 15 is a (3,5)-connected net with topology of (63)(69.8) whereas 16 is a (3,4)-connected framework with topology of (4.6.8)(4.62.83)(6.85)(62.83.10)(83). It should be noticed that titmb ligands adopt different conformation of cis-,trans-,trans-one in 15 (Fig. 5a),and both cis-,trans-,trans- and cis-,cis-,cis-ones in 16 (Fig. 5b and c). This study suggests that the different structures of 15 and 16 may arise from variable conformation of flexible ligand at different reaction temperatures since it is known that high temperature is beneficial for ligand to adopt the thermodynamically favored conformation with large activation barrier,and the low temperature favors the kinetic one. Therefore,the reaction temperature can be used to control the topology and dimensionality of the frameworks via thermodynamically and/or kinetically favored conformation [22]. Solvothermal reactions of Cd(OAc)2·2H2O (OAc- = acetate) with 3,30-azodibenzoic acid (H2L0) gave rise to three different Cd(II) frameworks {[Cd(L0)(DMF)2]·0.5DMF}n (17) in DMF at 100 ℃, [Cd(L0)(DMF)(MeOH)]n (18) in DMF-MeOH at 100 or 120 ℃,and {[Cd3(L0E˛ )3(DMF)4]·0.5DMF}n (19) in DMF at 120 ℃ [23]. MOFs 17- 19 exhibit the same 2D network but different structures with different coordination modes of the carboxylate groups and the different secondary building units (SBUs) of dinuclear [Cd2(L0)4(DMF)4] in 17,dinuclear [Cd2(L0)4(DMF)2(MeOH)2] in 18, and trinuclear [Cd3(L0)6(DMF)4] in 19.

|
Download:
|
| Fig. 5. Conformation of titmb ligand and coordination geometry of Co(II) in 15 (a) and 16 (b and c). | |
Another example is used to illustrate the influence of reaction temperature on the coordination ability of the central metal ions. The reaction of Na2WO4·2H2O,Cu(NO3)2·3H2O and phosphoric acid with 1,4-bis(pyrazol-1-ylmethyl)benzene (L00) at 130 and 150 ℃ produced [CuI3(L00)4][PW12O40] (20) and [CuI3(L00)4PW12O40] (21),respectively [24],with similar 2D network structures (Fig. 6). It is interesting that 20 and 21 display different cavity sizes of ca. 15.1Å × 17.2Å for 20 and ca. 14.8Å × 18.2Å for 21 when the reaction temperature is changed from 130 to 150 ℃,as a result,the polyoxometalate (POM) anions in 20 are stabilized by hydrogen bonds without coordination to the 2D network (Fig. 6a),while in 21 the POM anions coordinate with the Cu(I) ions (Fig. 6b). Therefore, the coordination number and coordination geometry of metal ions in 20 and 21 are different,it has been supposed that a high reaction temperature may be beneficial to increasing the coordination ability of central Cu(I) ions [24].

|
Download:
|
| Fig. 6. 2D networks of 20 (a) and 21 (b) with different cavity sizes. | |
The above mentioned examples are mainly the cases of starting from the same reactants,but obtaining MOFs with different structures under different reaction temperatures. Another type of the cases is the solid-to-solid structural transformation of MOFs as a function of temperature [25]. Typical examples are the singlecrystal to single-crystal (SCSC) transformations [26],which has evoked much research interest since such a pr℃ess can provide direct and useful information for designing new functional materials [27, 28]. The reported MOF’s SCSC transformations are realized by sorption/desorption or rearrangement of guest molecules [29] and both light-driven [30] and temperatureinduced changes [31]. The temperature-induced SCSC transformations of MOFs are mainly achieved by guest molecule desorption/ movement,sliding of layers,or cleavage and formation of bonds [32].
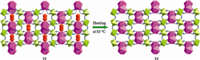
A porous 3d-4f bimetallic supramolecular 3D framework {[Nd(pyno)2(H2O)4][Fe(CN)6]·H2O}n (22) (pyno = pyridine-N-oxide) was formed by linking of the 1D chains through hydrogen bonding interactions [33]. When the as-synthesized 22 crystals were heated at 85 ℃ for 3 h under N2 atmosphere to remove the non-coordinated water molecules,{[Nd(pyno)2(H2O)4][Fe(CN)6]}n (23) was obtained. The results of crystal structure analysis show that there is significant framework contraction upon the removal of free water molecules (Fig. 7) [33]. The original framework can be regenerated by exposure of 23 to the water vapor with structural expansion. In addition,coordinated water molecules can be removed by further heating at 140 ℃,however,it causes the loss of single crystallinity at the same time [33]. This is an example of guest molecule induced SCSC structural transformation with framework flexibility and dynamicity,and is considered for possible application in sensors,actuators,and separation of the guest molecules.
|
Download:
|
| Fig. 7. SCSC transformation of 22-23 upon heating at 85 ℃. | |
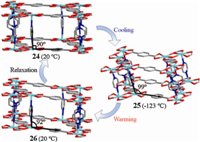
Temperature-induced reversible SCSC transformations reveal the framework flexibility as observed in the pillared-layer 3D framework [Cd2(nbdc)2(pyz)2]·4DMF (24) with rigid ligands of pyrazine (pyz) and 2-nitro-1,4-benzenedicarboxylate (nbdc2-). Crystallographic data show that the 3D framework distorted drastically upon cooling 24 from 20 ℃ to -123 ℃ with the lattice changing from orthorhombic (24,b = 90.08) to mon℃linic (25, b = 99.132(4)8) (Fig. 8) [34]. Furthermore,when the crystal was warmed up back to 293 K,a new but unstable mon℃linic phase 26 with b = 92.650(5)8 was found and will be turned back to 24 after more than 3 h without any further treatment (Fig. 8). This is an example of phase changes realized by slip and distortion upon heating/cooling without great change of 3D framework itself. The frameworks with such flexibility and dynamics may be responsive to external stimuli like heat,pressure,and guest molecules,and will have potentials for ‘‘smart’’ materials [35].
|
Download:
|
| Fig. 8. The temperature-induced SCSC structural transformation of 24,25 and 26. | |
The removal of coordinated solvent molecules may cause significant changes of the framework,such as structural transformation, structural reorganization and formation of higher dimensional structure via the coordination bond cleavage and formation. For instance,Sarma and Natarajan reported a SCSC transformation of 2D network [Cu3(μ3-OH)2(H2O)2(SIPA)(OAc)] (27) (SIPA2- = 5- sulphoisophthalate) to a 3D framework [Cu6(μ3-OH)4(SIPA) 2(OAc)2] (28) by heating at 180 ℃ [36]. It was found that the removal of coordinated water molecules upon heating makes the non-coordinated sulfonate oxygen atoms in 27 coordinate with the Cu(II) atoms in 28 as well as the dimensionality cross-over in the structure (Fig. 9a). In addition,the coordinated acetate is crucial in the observed SCSC transformation [36]. While in the case of MOFs with analogous 5-nitroisophthalate (NIPA2-) ligand,namely [Cu5(μ3-OH)2(H2O)6(NIPA)4]·5H2O (29) and [Cu5(μ2-OH)2(NIPA)4] (30),structural reorganization was observed by SCSC transformation. Upon heating 29 at 125 ℃,dehydration ℃curred. Interestingly, the pentameric Cu(II) clusters in MOF 29 split into trimeric (Cu3O12) and dimeric (Cu2O8) ones in 30 (Fig. 9b) [36]. SCSC transformation has also been observed in the Er(III) frameworks with benzimidazole-5,6-dicarboxylate ligand [37].
4. Conclusion and outlookThis paper outlined the influence of reaction temperature on the assembly of MOFs. It can be seen,on the one hand,that the reaction temperature has remarkable influence on the formation and structure of MOFs including the conformation,coordination mode and transformation of ligands; coordination ability of metal centers; the architecture topology,dimensionality and structural transformation and so on. On the other hand,it is also clear that the assembly,formation and structure of MOFs can differ greatly by changing temperature as well as other reaction conditions,which makes it difficult to predict and control the resulted MOFs,and thus further studies are needed. In addition,the mechanisms of temperature-driven behaviors still remain unexplored. Nevertheless, we hope that this review can provide some primary information to the design and construction of desired MOFs.
AcknowledgmentsThis work was financially supported by the National Natural ScienceFoundationofChina(Nos.91122001and21331002)andthe National Basic Research Program of China (No. 2010CB923303).
| [1] | S.L. James, Metal-organic frameworks, Chem. Soc. Rev. 32 (2003) 276-288. |
| [2] | (a) D.J. Tranchemontagne, J.L. Mendoza-Cortés, M. O'Keeffe, O.M. Yaghi, Secondary building units, nets and bonding in the chemistry of metal-organic frameworks, Chem. Soc. Rev. 38 (2009) 1257-1283;(b) M.J. Prakash, M.S. Lah, Metal-organic macrocycles, metal-organic polyhedral and metal-organic frameworks, Chem. Commun. (2009) 3326-3341. |
| [3] | (a) G. Férey, Hybrid porous solids: past, present, future, Chem. Soc. Rev. 37 (2008) 191-214;(b) O.M. Yaghi, M. O'Keeffe, N.W. Ockwig, et al., Reticular synthesis and the design of new materials, Nature 423 (2003) 705-714. |
| [4] | (a) R. Banerjee, A. Phan, B. Wang, et al., High-throughput synthesis of zeolitic imidazolate frameworks and application to CO2 capture, Science 319 (2008) 939-943;(b) X. Lin, A.J. Blake, C. Wilson, et al., A porous framework polymer based on a zinc(II) 4,40-bipyridine-2,6,20,60-tetracarboxylate: synthesis, structure, and "zeolite-like" behaviors, J. Am. Chem. Soc. 128 (2006) 10745-10753;(c) Z. Su, M. Chen, T.A. Okamura, et al., Reversible single-crystal-to-single-crystal transformation and highly selective adsorption property of three-dimensional cobalt (II) frameworks, Inorg. Chem. 50 (2011) 985-991;(d) S.S. Chen, M. Chen, S. Takamizawa, et al., Porous cobalt(II)-imidazolate supramolecular isomeric frameworks with selective gas sorption property, Chem. Commun. 47 (2011) 4902-4904. |
| [5] | (a) G.J. Halder, C.J. Kepert, B. Moubaraki, K.S. Murray, J.D. Cashion, Guest-dependent spin crossover in a nanoporous molecular framework material, Science 298 (2002) 1762-1765;(b) Z. Su, J. Fan, T.A. Okamura, W.Y. Sun, N. Ueyama, Ligand-directed and pHcontrolled assembly of chiral 3d-3d heterometallic metal-organic frameworks, Cryst. Growth Des. 10 (2010) 3515-3521;(c) Z. Su, J. Xu, J. Fan, et al., Synthesis, crystal structure, and photoluminescence of coordination polymers with mixed ligands and diverse topologies, Cryst. Growth Des. 9 (2009) 2801-2811. |
| [6] | (a) Y. Zhao, K. Chen, Y. Lu, et al., Structural modulation of silver complexes and their distinctive catalytic properties, Dalton Trans. 43 (2014) 2252-2258;(b) G.C. Lü, Y. Zhao, S.S. Chen, Z. Su, W.Y. Sun, J. Fan, Two-dimensional Mn(II) and Cd(II) networks with tetrazole-containing ligand and their properties, Inorg. Chem. Commun. 36 (2013) 59-62;(c) C. Hou, Q. Liu, P. Wang, W.Y. Sun, Porous metal-organic frameworks with high stability and selective sorption for CO2 over N2, Microporous Mesoporous Mater. 172 (2013) 61-66. |
| [7] | (a) Y. Zhao, M.F. Lü, J. Fan, et al., Syntheses, structures and photoluminescence properties of cadmium(II) and zinc(II) complexes with pyridinylcarboxamidecontaining ligand, Inorg. Chim. Acta 377 (2011) 138-143;(b) Y.Y. Liu, Y.Y. Jiang, J. Yang, Y.Y. Liu, J.F. Ma, Syntheses, structures and photoluminescence of zinc(II) and silver(I) coordination polymers based on 1,10-(1,4-butanediyl)bis(2-methylbenzimidazole) and different carboxylate ligands, CrystEngComm 13 (2011) 6118-6129;(c) Z. Su, K. Cai, J. Fan, et al., Cadmium(II) complexes with 3,5-di(1H-imidazol-1-yl)benzoate: topological and structural diversity tuned by counteranions, CrystEngComm 12 (2009) 100-108. |
| [8] | (a) C.P. Li, M. Du, Role of solvents in coordination supramolecular systems, Chem. Commun. 47 (2011) 5958-5972;(b) L.S. Long, pH effect on the assembly of metal-organic architectures, CrystEngComm 12 (2010) 1354-1365;(c) L. Luo, G.C. Lü, P. Wang, et al., pH-dependent cobalt(II) frameworks with mixed 3,30,5,50-tetra(1H-imidazol-1-yl)-1,10-biphenyl and 1,3,5-benzenetricarboxylate ligands: synthesis, structure and sorption property, CrystEngComm 15 (2013) 9537-9543;(d) D.F. Sun, S.Q. Ma, J.M. Simmons, et al., An unusual case of symmetry-preserving isomerism, Chem. Commun. 46 (2010) 1329-1331. |
| [9] | (a) V. Iancu, A. Deshpande, S.W. Hia, Manipulating Kondo temperature via single molecule switching, Nano Lett. 6 (2006) 820-823;(b) F. Luo, M.B. Luo, Y.H. Liu, Temperature-controlled structure diversity observed in the Zn(II)-oxalate-4,40-bipyridine three-member system, CrystEngComm 12 (2010) 1750-1753;(c) G.C. Xu, Y.J. Ding, T.A. Okamura, et al., Structure diversity and reversible anion exchange properties of cadmium(II) complexes with 1,3,5-tris(imidazol-1-ylmethyl)benzene: counteranion-directed flexible ligand conformational variation, CrystEngComm 10 (2008) 1052-1062. |
| [10] | (a) D. Liu, Z.G. Ren, H.X. Li, et al., pH-dependent solvothermal formation of two different 3D multiple interpenetrating nets from the same components of Zn(NO3)2, 1,3-benzenedicarboxylate and 1,4-bis[2-(4-pyridyl)ethenyl]benzene, CrystEngComm 12 (2010) 1912-1919;(b) S.S. Chen, Z.S. Bai, J. Fan, et al., Synthesis and characterization of metal complexes with a mixed 4-imidazole-containing ligand and a variety of multicarboxylic acids, CrystEngComm 12 (2010) 3091-3104. |
| [11] | G.P. Yang, L. Hou, L.F. Ma, Y.Y. Wang, Investigation on the prime factors influencing the formation of entangled metal-organic frameworks, CrystEngComm 15 (2013) 2561-2578. |
| [12] | (a) M. Chen, M.S. Chen, T.A. Okamura, et al., A series of silver(I)-lanthanide(III) heterometallic coordination polymers: syntheses, structures and photoluminescent properties, CrystEngComm 13 (2011) 3801-3810;(b) C.A. Bauer, T.V. Timofeeva, T.B. Settersten, et al., Influence of connectivity and porosity on ligand-based luminescence in zinc metal-organic frameworks, J. Am. Chem. Soc. 129 (2007) 7136-7144;(c) P. Mahata, A. Sundaresanb, S. Natarajan, The role of temperature on the structure and dimensionality of MOFs: an illustrative study of the formation of manganese oxy-bis(benzoate) structures, Chem. Commun. (2007) 4471-4473. |
| [13] | (a) S. Bauer, C. Serre, T. Devic, et al., High-throughput assisted rationalization of the formation of metal organic frameworks in the iron(III) aminoterephthalate solvothermal system, Inorg. Chem. 47 (2008) 7568-7576;(b) L.F. Ma, L.Y. Wang, D.H. Lu, S.R. Batten, J.G. Wang, Structural variation from 1D to 3D: effects of temperature and pH value on the construction of Co(II)-H2tbip/bpp mixed ligands system, Cryst. Growth Des. 9 (2009) 1741-1749;(c) E.C. Yang, T.Y. Liu, Q. Wang, X.J. Zhao, Temperature-controlled assembly of two fluorescent ZnII polymers from 3D pillared-layer framework to 2D (4,4) layer, Inorg. Chem. Commun. 14 (2011) 285-287. |
| [14] | P.M. Forster, A.R. Burbank, C. Livage, G. Férey, A.K. Cheetham, The role of temperature in the synthesis of hybrid inorganic-organic materials: the example of cobalt succinates, Chem. Commun. (2004) 368-369. |
| [15] | M. Dan, C.N.R. Rao, A building-up process in open-framework metal carboxylates that involves a progressive increase in dimensionality, Angew. Chem. Int. Ed. 45 (2006) 281-285. |
| [16] | (a) P.J. Calderone, D. Banerjee, A.M. Plonka, S.J. Kim, J.B. Parise, Temperature dependent structure formation and photoluminescence studies of a series of magnesiuμ-based coordination networks, Inorg. Chim. Acta 394 (2013) 452-458;(b) P. Wang, L. Luo, J. Fan, et al., Syntheses, structures, sorption and magnetic properties of copper (II) frameworks with varied topologies, Microporous Mesoporous. Mater. 175 (2013) 116-124;(c) L. Luo, K. Chen, Q. Liu, et al., Zinc(II) and cadmium(II) complexes with 1,3,5-benzenetricarboxylate and imidazole-containing ligands: structural variation via reaction temperature and solvent, Cryst. Growth Des. 13 (2013) 2312-2321. |
| [17] | Z. Su, J. Fan, T. Okamura, et al., Interpenetrating and self-penetrating zinc(II) complexes with rigid tripodal imidazole-containing ligand and benzenedicarboxylate, Cryst. Growth Des. 10 (2010) 1911-1922. |
| [18] | (a) H. Chun, D.N. Dybtsev, H. Kim, K. Kim, K. Synthesis, X-ray crystal structures, and gas sorption properties of pillared square grid nets based on paddle-wheel motifs: implications for hydrogen storage in porous materials, Chem. Eur. J. 11 (2005) 3521-3529;(b) B.Q. Ma, K.L. Mulfort, J.T. Hupp, Microporous pillared paddle-wheel frameworks based on mixed-ligand coordination of zinc ions, Inorg. Chem. 44 (2005) 4912-4914;(c) X.L. Wang, C. Qin, E.B. Wang, et al., An unprecedented eight-connected selfpenetrating network based on pentanuclear zinc cluster building blocks, Chem. Commun. (2005) 4789-4791. |
| [19] | H.L. Jiang, Y. Tatsu, Z.H. Lu, Q. Xu, Non-, micro-, and mesoporous metal-organic framework isomers: reversible transformation, fluorescence sensing, and large molecule separation, J. Am. Chem. Soc. 132 (2010) 5586-5587. |
| [20] | (a) C. Livage, C. Egger, G. Férey, Hydrothermal versus nonhydrothermal synthesis for the preparation of organic-inorganic solids: the example of cobalt(II) succinate, Chem. Mater. 13 (2001) 410-414;(b) Z.S. Bai, Z.P. Qi, Y. Lu, Q. Yuan, W.Y. Sun, Novel inorganic-organic hybrid frameworks of manganese(II): syntheses, crystal structures, and physical properties, Cryst. Growth Des. 8 (2008) 1924-1931. |
| [21] | (a) J.K. Sun, W. Li, L.X. Cai, J. Zhang, Structural diversity of the mixed-ligand system Mn-cpdba-2,20-bpy controlled by temperature, CrystEngComm 13 (2011) 1550-1556;(b) T.L. Hu, Y. Tao, Z. Chang, X.H. Bu, Zinc(II) complexes with a versatile multitopic tetrazolate-based ligand showing various structures: impact of reaction conditions on the final product structures, Inorg. Chem. 50 (2011) 10994-11003;(c) S.M. Zhang, T.L. Hu, J.L. Du, X.H. Bu, Tuning the formation of copper(I) coordination architectures with quinoxaline-based N,S-donor ligands by varying terminal groups of ligands and reaction temperature, Inorg. Chim. Acta 362 (2009) 3915-3924. |
| [22] | (a) G.X. Liu, H. Xu, H. Zhou, S. Nishiharab, X.M. Ren, Temperature-induced assembly of MOF polymorphs: syntheses, structures and physical properties, CrystEngComm 14 (2012) 1856-1864. |
| [23] | L.L. Liu, L. Liu, J.J. Wang, Solvent-and temperature-driven synthesis of three Cd(II) coordination polymers based on 3,3'-azodibenzoic acid ligand: crystal structures and luminescent properties, Inorg. Chim. Acta 397 (2013) 75-82. |
| [24] | G.F. Hou, L.H. Bi, B. Li, L.X. Wu, Reaction controlled assemblies of polyoxotungstates(-molybdates) and coordination polymers, Inorg. Chem. 49 (2010) 6474-6483. |
| [25] | (a) J.P. Ma, Y.B. Dong, R.Q. Huang, M.D. Smith, C.Y. Su, Spontaneously resolved chiral three-fold interpenetrating diamondoidlike Cu(II) coordination polymers with temperature-driven crystal-to-crystal transformation, Inorg. Chem. 44 (2005) 6143-6145;(b) L.Z. Zhang, W. Gu, Z.L. Dong, X. Liu, B. Li, Phase transformation of a rare-earth Anderson polyoxometalate at low temperature, CrystEngComm 10 (2008) 1318-1320. |
| [26] | (a) J.J. Vittal, Supramolecular structural transformations involving coordination polymers in the solid state, Coord. Chem. Rev. 251 (2007) 1781-1795;(b) X.N. Cheng, W.X. Zhang, X.M. Chen, Single crystal-to-single crystal transformation from ferromagnetic discrete molecules to a spin-canting antiferromagnetic layer, J. Am. Chem. Soc. 129 (2007) 15738-15739;(c) M. Nagarathinam, J.J. Vittal, Anisotropic movements of coordination polymers upon desolvation: solid-state transformation of a linear 1D coordination polymer to a ladderlike structure, Angew. Chem. Int. Ed. 45 (2006) 4337-4341. |
| [27] | (a) J.P. Zhang, Y.Y. Lin, W.X. Zhang, X.M. Chen, Temperature-or guest-induced drastic single-crystal-to-single-crystal transformations of a nanoporous coordination polymer, J. Am. Chem. Soc. 127 (2005) 14162-14163;(b) L. Pan, H. Liu, X. Lei, et al., RPM-1: a recyclable nanoporous material suitable for ship-in-bottle synthesis and large hydrocarbon sorption, Angew. Chem. Int. Ed. 42 (2003) 542-546;(c) M.P. Suh, H.R. Moon, E.Y. Lee, S.Y. Jang, A redox-active two-dimensional coordination polymer: preparation of silver and gold nanoparticles and crystal dynamics on guest removal, J. Am. Chem. Soc. 128 (2006) 4710-4718. |
| [28] | J. Li, P. Huang, X.R. Wu, et al., Metal-organic frameworks displaying single crystalto-single crystal transformation through postsynthetic uptake of metal clusters, Chem. Sci. 4 (2013) 3232-3238. |
| [29] | (a) P.S. Mukherjee, N. Lopez, A.M. Arif, F. Cervantes-Lee, J.C. Noveron, Singlecrystal to single-crystal phase transitions of bis(N-phenylisonicotinamide)silver( I) nitrate reveal cooperativity properties in porous molecular materials, Chem. Commun. 14 (2007) 1433-1435;(b) M.H. Mir, J.J. Vittal, Single-crystal to single-crystal transformation of cyclic water heptamer to another (H2O)7 cluster containing cyclic pentamer, Cryst. Growth Des. 8 (2008) 1478-1480. |
| [30] | H. Konaka, L.P. Wu, M. Munakata, et al., Syntheses and structures of photochromic silver(I) coordination polymers with cis-1,2-dicyano-1,2-bis(2,4,5-trimethyl-3-thienyl)ethene, Inorg. Chem. 42 (2003) 1928-1934. |
| [31] | (a) D.K. Kumar, D.A. Jose, A. Das, P. Dastidar, From diamondoid network to (4,4) net: effect of ligand topology on the supramolecular structural diversity, Inorg. Chem. 44 (2005) 6933-6935;(b) S. Yahyaoui, W. Rekik, H. Naili, T. Mhiri, T. Bataille, Synthesis, crystal structures, phase transition characterization and thermal decomposition of a new dabcodiium hexaaquairon(II) bis(sulfate): (C6H14N2)[Fe(H2O)6](SO4)2, J. Solid State Chem. 180 (2007) 3560-3570;(c) G. Mahmoudi, A. Morsali, Crystal-to-crystal transformation from a weak hydrogen-bonded two-dimensional network structure to a two-dimensional coordination polymer on heating, Cryst. Growth Des. 8 (2008) 391-394;(d) N.L. Toh, M. Nagarathinam, J.J. Vittal, Topochemical photodimerization in the coordination polymer [{(CF3CO2)(μ-O2CCH3)Zn}2(μ-bpe)2]n through singlecrystal to single-crystal transformation, Angew. Chem. Ent. Ed. 44 (2005) 2237-2241. |
| [32] | (a) C.D. Wu, W.B. Lin, Highly porous, homochiral metal-organic frameworks: solvent-exchange-induced single-crystal to single-crystal transformations, Angew. Chem. Int. Ed. 44 (2005) 1958-1961;(b) T.K. Maji, G. Mostafa, R. Matsuda, S. Kitagawa, Guest-induced asymmetry in a metal-organic porous solid with reversible single-crystal-to-single-crystal structural transformation, J. Am. Chem. Soc. 127 (2005) 17152-17153;(c) J. Sun, F.N. Dai, W.B. Yuan, et al., Dimerization of a metal complex through thermally induced single-crystal-to-single-crystal transformation or mechanochemical reaction, Angew. Chem. Int. Ed. 50 (2011) 7061-7064. |
| [33] | K.L. Gurunatha, G. Mostafa, D. Ghoshal, T.K. Maji, Single-crystal-to-single-crystal structural transformation in a three-dimensional bimetallic (4f-3d) supramolecular porous framework, Cryst. Growth Des. 10 (2010) 2483-2489. |
| [34] | X.F. Wang, Y. Wang, Y.B. Zhang, et al., Layer-by-layer evolution and a hysteretic single-crystal to single-crystal transformation cycle of a flexible pillared-layer open framework, Chem. Commun. 48 (2012) 133-135. |
| [35] | (a) S. Kitagawa, K. Uemura, Dynamic porous properties of coordination polymers inspired by hydrogen bonds, Chem. Soc. Rev. 34 (2005) 109-119;(b) D. Bradshaw, J.E. Warren, M.J. Rosseinsky, Reversible concerted ligand substitution at alternating metal sites in an extended solid, Science 315 (2007) 977-980;(c) D.N. Dybtsev, H. Chun, K. Kim, Rigid and flexible: a highly porous metalorganic framework with unusual guest-dependent dynamic behavior, Angew. Chem. Ent. Ed. 43 (2004) 5033-5036. |
| [36] | D. Sarma, S. Natarajan, Usefulness of in situ single crystal to single crystal transformation (SCSC) studies in understanding the temperature-dependent dimensionality cross-over and structural reorganization in copper-containing metal-organic frameworks (MOFs), Cryst. Growth Des. 11 (2011) 5415-5423. |
| [37] | X.J. Hong, M.F. Wang, H.G. Jin, et al., Single-crystal to single-crystal transformation from a 1-D chain-like structure to a 2-D coordination polymer on heating, CrystEngComm 15 (2013) 5606-5611. |




