b Xiangya Hospital of Central South University, Changsha 410008, China
1. Introduction
Selaginella involven Spring is abundantly distributed in south China. It has been used as a folk herbal medicine for its hemostasis, antitumor,antibacterial,anti-inflammation,antiviral activities and treatment of cardiovascular diseases [1, 2, 3]. A number of biflavonoids, lignans,alkaloids and anthraquinones have been identified from genus Selaginella [4, 5, 6]. However,few terpenoids were reported [7]. In our study on chemical constituents of the genus Selaginella involven Spring,here we report the isolation and structural elucidation of two new compounds named as 3β,12,16- trihydroxy-6,8,11,13-abietatrien (1),(8R,8'S)-4,4',8-trihydroxyl- 3,3'-dimethoxyl-9'-lignanolide (2) and a new natural product 4,4'-dihydroxyl-3,3',5,5'-dimethoxyldiphenyl diketone (3). Abietane diterpenoid is also isolated for the first time from genus Selaginella. 2. Experimental
Herbs of Selaginella involven Spring were collected from Mao’er Mountain,Guangxi Zhuang Autonomous Region,China,in June 2009,and authenticated by Prof. Zhenji Li (Xia Men University,Xia Men,China). A voucher specimen (No. 20090617) was deposited in School of Pharmaceutical Sciences,Central South University.
The whole herbs of Selaginella involven Spring (10.0 kg) were refluxed in 70% EtOH twice (100 L,80 L,2 h each time). After the removal of the solvent under reduced pressure,the extract (0.8 kg) was subjected to polyamide with a gradient elution of H2O,30%, 50%,70%,95% EtOH to afford five fractions. The 50% EtOH portion was subjected to silica gel column chromatography with a gradient elution of CHCl3/acetone (100:0-100:10) to obtain eight subfractions (S1-S8). Separation of sub-fraction S5 on Sephadex LH-20 (MeOH/H2O in gradient) and Pre-HPLC (YMC-Pack ODS-A; 250 mm × 10 mm,10 μm; 3 mL/min; 35% ACN/H2O) yielded compound 1 (10 mg),compound 2 (20 mg) and compound 3 (30 mg),respectively.
The high differentiated PC-12 cells were seeded at constant density (1 × 104/cm2) and cultured in DMEM,supplemented respectively with 10% fetal bovine serum,penicillin (100 IU/mL), streptomycin (100 mg/mL) and L-glutamine (2 mmol/L). To establish the H/R model,PC-12 cells were subjected to 5 h of hypoxia (N2/CO2,95:5) in pre-conditioned hypoxic medium (serum free DMEM without glucose and sodium pyruvate,which was incubated under hypoxic condition for 2 h) followed by 20 h of reoxygenation. Hypoxic medium was replaced with fresh medium upon switching to reoxygenation. The compounds extracted were added into media to explore its effect on H/R-induced cellular injury. At the end of experiments,cells were collected for analysis of cellular apoptosis. 3. Results and discussion
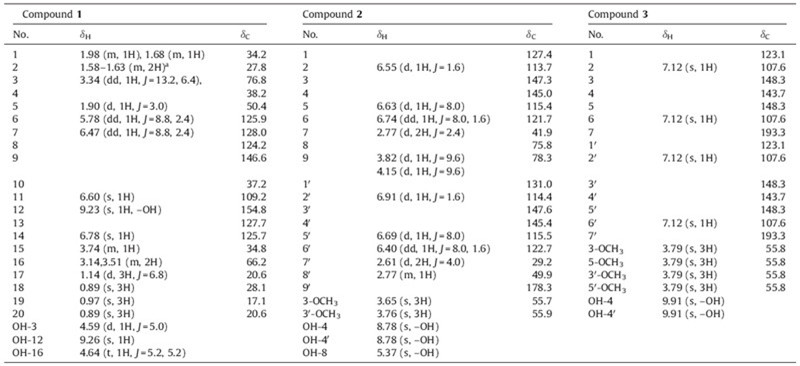
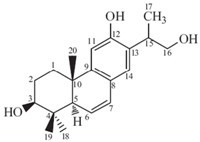
Compound 1 was obtained as an amorphous yellow powder. Its molecular formula was determined as C20H28O3 by HREIMS (m/z 316.2033; cacld. 316.2033). The IR spectrum revealed the presence of hydroxyl (3405 cm-1),aromatic (1600,1560 and 1457 cm-1) groups. The absorption band at 278 nm in the UV spectrum suggested the presence of an aromatic ring. The 1H NMR spectrum of compound 1 (Table 1) displayed the presence of four tertiary methyl groups (δH 0.89,0.89,0.97,1.14),three methylene groups at δ 3.14,3.51 (m,2H),1.97-2.00 (m,1H),1.66-1.69 (m,1H),1.58- 1.63 (m,2H),three methine groups at δ 3.74 (m,1H),3.34 (dd,1H, J = 13.2 Hz,6.4 Hz) and 1.90 (d,1H,J = 3.0 Hz),two cis olefinic protons at δ 5.78 (dd,1H,J = 8.8,2.4 Hz) and 6.47(dd,1H,J = 8.8, 2.4 Hz),two singlet aromatic protons at δ 6.60 and 6.78. The 13C NMR and DEPT spectra suggested that compound 1 (Table 1) is a diterpenoid derivative with a total of twenty carbons [8, 9, 10], consisting of four methyls (δC 17.1,20.6,20.6,28.1),three methylenes (δC 27.8,34.2,66.2),seven methines (δC 34.8, 50.4,76.8,109.2,125.7,125.9,128.0),two sp3 quaternary carbons (δC 37.2,38.2),four sp2 quaternary carbons (δC 124.2,127.7,146.6, 154.8). The HMBC spectrum allowed us to construct compound 1 as an abietane-type derivative with trihydroxy group at C-3,C-12 and C-16. The structure is very close to that of 6-dehyohinokiol [8, 9],but markedly different in C-16 with a hydroxyl group. The correlations from H-15,H-16,and Me-17 to C-13 suggested that C- 15 is in the middle of the three-carbon chain fragment C-16-C- 15-C-17. The correlations from Me-18 and Me-19 to the C-3 carbonyl carbon establsihed the linkages of the C-5,C-4 and C-3. H- 3 appeared doublets of doublets and had coupling constants of 6.4 and 13.2 Hz. These facts indicated a typical 3β-hydroxyl substituted in A ring [10, 11]. In the modified Mosher’s method,since the biogenetically related diterpnoids have a trans-chair conformation, the A and B rings,the C-20 methyl group,and the C-5 proton were presumed to be trans-diaxial [10]. From these data,the structure of compound 1 (Fig. 1) was determined as 3β,12,16-trihydroxy-6,8,11,13-abietatrien. The full assignment of 1H NMR and 13C NMR spectra was accomplished with the aid of DEPT,HSQC,1H-1H COSY and HMBC experiments (Fig. 2).
| Table 1 1H NMR (400 MHz) and 13C NMR(100MHz) data of compound 1-3 in DMSO-d6 (δ in ppm,J in Hz). |
|
Download:
|
| Fig. 1.The structure of compound 1. | |
|
Download:
|
| Fig. 2.Key 1H-1H COSY and HMBC correlations of compound 1. | |
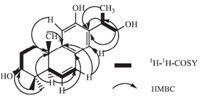
Compound 2 was obtained as a colorless solid. Its molecular formula was determined as C20H22O7 by HREIMS (m/z 374.1350; cacld. 374.1360). The IR spectrum revealed the presence of hydroxyl (3325 cm-1),aromatic (1612,1560 and 1512 cm-1) and carbonyl (1748 cm-1) groups. Its UV spectrum showed the maximum absorption bands at 228 nm and 282 nm. The 1H NMR spectrum of compound 2 (Table 1) showed six aromatic protons at δ 6.91 (d,1H,J = 1.6 Hz),6.74 (dd,1H,J = 8.0 Hz,1.6 Hz),6.69 (d,1H, J = 8.0 Hz),6.63 (d,1H,J = 8.0 Hz),6.54 (d,1H,J = 1.6 Hz),6.40 (dd, 1H,J = 8.0 Hz,1.6 Hz),as two ABX systems. Additionally,three CH2 groups at δ 4.15 (d,1H,J = 9.6 Hz) and δ 3.82 (d,1H,J = 9.6 Hz),d 2.77 (d,2H,J = 2.4 Hz) and δ 2.61 (d,2H,J = 4.0 Hz),and a CH δ 2.77 (m,1H),two aromatic methoxy groups at δ 3.76 (s,3H) and δ 3.65 (s,3H),were observed. The 13C NMR and DEPT,HSQC spectrum showed the presence of a lactone C=O at δ 178.3 (C-9'),three CH2 groups at δ 78.3 (C-9),41.9 (C-7),29.2 (C-7'),and a CH groups at δ 49.9 (C-8'),two methoxy carbons at δ 55.7 and δ 55.9,as well as carbon signals of two aromatic rings (δ 113.6-147.6). From the analysis of HMBC and 1H-1H COSY,compound 2 is structurally very close to wikstromol [12],which was also isolated by us from Selaginella involven Spring. There was a marked difference in C-9 Chemical shift in the two compounds. In wikstromol this signal was at δ 70.3 (C-9),while in compound 2 it was at δ 78.3 (C-9). In the HMBC spectrum,C-1',C-9',C-7 showed correlations with H-8', which indicated that the hydroxyl substituent was at C-8 not C-8'. In addition,the signals of C-7 correlated with H-2,H-6,which indicated that C-7 was substituted by an aromatic ring and a lactone ring. The signal of C-7' correlated with H-2',H-6',which indicated that C-7' was substituted by a aromatic ring and a lactone ring. The CD spectrum exhibited a positive cotton effect at 228 nm and 276 nm [13, 14, 15]. From these data,the structure of compound 2 (Fig. 3) was identified as (8R,8'S)-4,40,8-trihydroxyl-3,3'- dimethoxyl-90 lignanolide (Fig. 4).
|
Download:
|
| Fig. 3.The structure of compound 2. | |
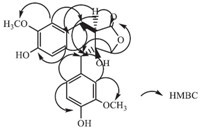
|
Download:
|
| Fig. 4.Key 1H-1H COSY and HMBC correlations of compound 2. | |
Compound 2 was obtained as light yellow acicular crystals. Its molecular formula was determined as C18H18O8 by HREIMS (m/z 362.0984; cacld. 362.0996). The IR spectrum revealed the presence of hydroxyl (3325 cm-1),carbonyl (1678 cm-1),aromatic (1612, 1560 and 1512 cm-1) groups. Its UV spectrum showed a maximum absorption band at 330 nm. The 1H NMR spectrum of compound 2 (Table 1) showed two 3,4,5-trisubstituted benzenes at δ 7.12(s, 4H),four aromatic methoxy groups at δ 3.79 (s,12H). The 13C NMR and DEPT,HSQC spectrum showed the presence of two C=O at δ 193.3,four methoxy carbons at δ 55.8,as well as carbon signals of two aromatic rings (δ 107.6-148.3). In the HMBC spectrum,two C=O groups showed correlations with H-2,H-6 and H-2',H-6', respectively. From these data,the structure of compound 2 (Fig. 5) was identified as 4,4'-dihydroxyl-3,3',5,5'-dimethoxyldiphenyldiketone (Figs. 6 and 7).
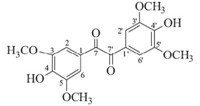
|
Download:
|
| Fig. 5.The structure of compound 3. | |
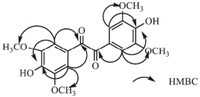
|
Download:
|
| Fig. 6.Key 1H-1H COSY and HMBC correlations of compound 3. | |
|
Download:
|
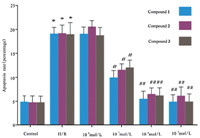
| Fig. 7.Effect of compounds on PC-12 cells induced by hypoxia/reoxygenation. *: Compared with the control group,p < 0.05; #: Compared with H/R,p < 0.05; H/R: hypoxia/reoxygenation | |
Additionally,we investigated the protective effect of compounds on the injury of PC-12 cells induced by hypoxia/ reoxygenation [16, 17]. These three compounds displayed potent protective effect on PC-12 cells induced by hypoxia/reoxygenation at 10-5 mol/L,10-6 mol/L and 10-7 mol/L (Fig. 3) with good dose dependency. We will continue to conduct pharmacological activity evaluations on the three compounds and explore the underlying mechanism of action. 4. Conclusion
In this study on the chemical constituents of the genus Selaginella involven Spring,we reported the isolation and structural elucidation of two new compounds named as 3β,12,16-trihydroxy- 6,8,11,13-abietatrien (1),(8R,80S)-4,4',8-trihydroxyl-3,3'- dimethoxyl-9'-lignanolide (2) and a new natural product 4,4'- dihydroxyl-3,3',5,5'-dimethoxyldiphenyldiketone (3) along with their potent protective effect against the injury of PC-12 cells induced by hypoxia/reoxygenation for the first time.
AcknowledgmentsThis research was supported by National Natural Science Foundation (No. 31370370),the Doctoral Program Foundation of Institutions of Higher Education of China (No. 20100162110057), Chinese Medicine Research Program of Hunan Province (Nos. 2009059 and 2010004).
| [1] | M.X. Lu, K.L. Huang, S.Y. Shi, H. Zhang, Study on the chemical constituents of Selaginella invovens Spring and antibaceterial activity, Nat. Prod. Res. Dev. 21 (2009) 973-975. |
| [2] | K.L. Chen, G.W. Plumb, R.N. Bennett, Y. Bao, Antioxidant activities of extracts from five anti-viral medicinal plants, J. Ethnopharmacol. 96 (2005) 201-205. |
| [3] | C.J. Wang, C.P. Hu, K.P. Xu, et al., Protective effect of selaginellin on glutamateinduced cytotoxicity and apoptosis in differentiated PC12 cells, N-S Arch. Pharmacol. 381 (2010) 73-81. |
| [4] | R.C. Swamy, O. Kunert, W. Schühly, et al., Structurally unique biflavonoids from Selaginella chrysocaulosand and Selaginella bryopteris, Chem. Biodivers. 3 (2006) 405-413. |
| [5] | S.H. Qi, S. Zhang, J.S. Huang, et al., Glycerol derivatives and sterols from Sargassum parvivesiculosum, Chem. Pharm. Bull. 52 (2004) 986-988. |
| [6] | J.F. Liu, K.P. Xu, D.J. Jiang, et al., A new flavonoid from Selaginella tamariscina, Chin. Chem. Lett. 20 (2009) 595-597. |
| [7] | X. Wang, Z.L. Li, L.L. Gao, J.N. Li, H.M. Hua, Chemical constituents of Selaginella tamariscina Spring, Shenyang Pharm. Univ. 126 (2009) 623-625. |
| [8] | C.F. Chyu, H.C. Lin, Y.H. Kuo, New abietane and seco-abietane diterpenes from the root of Taiwania cryptomerioides, Chem. Pharm. Bull. 53 (2005) 11-14. |
| [9] | W. Yuan, Z.M. Lu, Y. Liu, et al., Three new podocarpane-type diter penoids from Callus of Securinega, Chem. Pharm. Bull. 53 (2005) 1610-1612. |
| [10] | S.S. Liu, H.L. Zhang, S.W. Zhang, et al., Abietane diterpenoids from Clerodendrum bungei, J. Nat. Prod. 71 (2008) 755-759. |
| [11] | Y. Inaba, T. Hasuda, Y. Hitotsuyangagi, et al., Abietane diterpenoids and a sesquiterpene pyridine alkaloid from Euonymus lutchuensis, J. Nat. Prod. 76 (2013) 1085-1090. |
| [12] | F. Kawmura, S. Kawai, H. Ohashi, Sesquilignans and lignans from Tsuga heterophylla, Phytochemistry 44 (1997) 1351-1357. |
| [13] | L.M.X. Lopes, M. Yoshida, O.R. Gottlieb, Dibenzylbutyrolactone lignans from Virola sebifera, Phytochemistry 22 (1983) 1516-1518. |
| [14] | B.R. Prabhu, N.B. Mulchandani, Lignans from Piper cubeba, Phytochemistry 24 (1985) 329-331. |
| [15] | B.N. Su, W.P. Jones, M. Cuendet, et al., Constituents of the stems of macrococculus pomiferus and their inhibitory activities against cyclooxygenases-1and-2, Phytochemistry 65 (2004) 2861-2866. |
| [16] | J.X. Zheng, Y. Zheng, H. Zhi, et al., New 3',8"-linked biflavonoids from Selaginella uncinata displaying protective effect against anoxia, Molecules 16 (2011) 6206- 6214. |
| [17] | C.T. Li, W.P. Zhang, S.H. Fang, et al., Baicalin attenuates oxygen-glucose deprivation induced injury by inhibiting oxidative stress-mediated 5-lipoxygenase activation in PC12 cells, Acta Pharm. Sin. 31 (2010) 137-144. |